Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States. According to the American Cancer Society, an estimated 32,180 individuals in the United States will be diagnosed with pancreatic cancer in 2005.[1] This disease is associated with a high mortality rate; the 5-year survival rate is estimated to be 4%. Currently, surgical resection is the only option for a cure. Unfortunately, due to its late presentation, resection is possible in only 15% of cases of pancreatic cancer. Even in this population, the 5-year survival rate is approximately 20%.[2,3] When disease is unresectable, chemotherapy, radiation therapy, or a combination of these modalities may be used to increase overall quality of life. Careful preoperative staging is paramount in the determination of optimal treatment, surgical intervention, or palliation of these patients. The primary goals of staging are to identify patients likely to benefit from surgery (by complete, margin-negative resection), provide prognostic information, and to obtain a tissue diagnosis in patients unsuitable for surgery or when otherwise needed.
The regional anatomy of the pancreas is complex, making procurement of cytologic samples historically difficult without exploratory laparotomy. Traditionally, computed tomography (CT) or endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has been used to obtain biopsies of the pancreas. However, not all lesions are accessible due to surrounding organs and vasculature. Additionally, these techniques are associated with a risk of peritoneal dissemination of cancer cells and have a false-negative rate of up to 20%.[4,5] Endoscopic retrograde cholangiopancreatography (ERCP) brush cytology has a false-negative rate of at least 30%.[6]
EUS was developed in the 1980s to improve the imaging of the pancreas. Traditional transabdominal ultrasound imaging of the pancreas is hampered by intervening bowel gas, bone, and fat. By placing a high-frequency transducer directly within the stomach or duodenal lumen, EUS can obtain a detailed image of the pancreas that has a higher resolution than CT scan or magnetic resonance imaging, but with a much narrower field of view. These high-resolution images allow for identification of lesions as small as 2–3 mm and involvement of adjacent vascular structures.
This report specifically addresses the role of EUS-FNA in the detection and confirmation of pancreatic cancer.
EUS-FNA in Pancreatic Malignancy
EUS has become an important tool in the armamentarium of gastroenterologists for the diagnosis and staging of pancreatic neoplasms. EUS alone is limited in its ability to discriminate between malignant and benign processes. EUS-FNA, with its ability to obtain a tissue diagnosis, has increased the accuracy of EUS in the diagnosis and staging of pancreatic malignancies. The sensitivity, specificity, and accuracy of EUS-FNA for pancreatic lesions range from 64% to 94%, 71% to 100%, and 78% to 95%, respectively (Table).[7–18]
Table.
Sensitivity, Specificity, and Accuracy of EUS-FNA of Pancreatic Masses
| Study | Sensitivity | Specificity | Accuracy | # of Cases |
|---|---|---|---|---|
| Raut et al[7] | 91% | 100% | 92% | 233 |
| Eloubeidi et al[8] | 84% | 97% | 84% | 158 |
| Harewood et al[9] | 94% | 71% | 92% | 185 |
| Ylgan et al[10] | 78% | 100% | NR | 80 |
| Chang et al[11] | 92% | 100% | 95% | 44 |
| Bhutani et al[12] | 64% | 100% | NR | 47 |
| Williams et al[13] | 82% | 100% | 85% | 144 |
| Binmoeller et al[14] | 76% | 100% | NR | 40 |
| Gress et al[15] | 80% | 100% | 85% | 121 |
| Voss et al[16] | 75% | 88% | 74% | 73 |
| Fritscher-Ravens et al[17] | 84% | 100% | 92% | 78 |
| Afify et al[18] | 80% | 89% | NR | 69 |
NR = Not reported
The presence of changes of chronic pancreatitis limits the imaging of the pancreas and complicates the interpretation of findings. In these cases, differentiation between small focal areas of pancreatitis and malignancy is difficult due to the similar appearance. In the case of advanced chronic pancreatitis, calcifications can create acoustic shadowing that may limit clear views of the pancreatic parenchyma. Not surprisingly, the sensitivity of EUS-FNA for the detection of pancreatic malignancies in the setting of chronic pancreatitis is 54% vs 89% in patients with an otherwise normal-appearing pancreas.[19]
Lymph node staging and the detection of metastatic lesions are essential aspects of pancreatic cancer staging. EUS-FNA allows for sampling of suspicious-appearing lymph nodes and liver lesions. EUS-FNA has been shown to increase the accuracy of lymph node staging and thereby reduce the number of unnecessary operations.[11] The left lobe and the inferior aspect of the right lobe of the liver can be well visualized by EUS, and this has led to the detection of previously unseen small liver lesions. An immediate EUS-FNA of the liver lesion can be performed to detect metastatic disease, which would preclude surgical intervention. This procedure is considered to be safe. In a series of 167 EUS-FNAs of liver lesions, the reported complication rate was 4%. There was 1 death in the setting of an occluded biliary stent and subsequent cholangitis.[20] It is reasonable to give antibiotic prophylaxis in conjunction with biliary drainage at the time of EUS-FNA of liver lesions to minimize complications in the setting of obstructing pancreatic tumors, although no data exist to prove that this approach is beneficial.
Impact of EUS-FNA
Accurate staging of pancreatic malignancies is essential to avoid the expense, morbidity, and mortality related to unnecessary surgery. Analysis of the impact of EUS on the cost and management of pancreatic cancer revealed that EUS in addition to helical CT reduced the overall cost of care by nearly $33,000. This was primarily due to avoiding unnecessary surgical exploration.[21] The downside of an EUS-based strategy is overstaging. When EUS alone was used to determine resectability, approximately 2% of patients were incorrectly deemed unresectable and 4% of patients underwent “unnecessary” laparotomy, in which the patient was found to be unresectable at the time of exploration. In contrast, reliance on CT alone would have caused 1% of patients to be misclassified as unresectable and 22% of patients to be deemed unresectable at the time of exploration.[21]
Controversies in EUS-FNA of Pancreatic Lesions
There is a general consensus that EUS-FNA is reasonable to perform in patients who are poor surgical candidates or are deemed unresectable because it will aid in the medical management of that patient. However, the issue of performing EUS-FNA in patients who are potentially resectable is more controversial. Some experts argue that the EUS-FNA results would not alter management, and thus is an unnecessary procedure. This argument is supported by the fact that the sensitivity of EUS-FNA is in the range of 80% to 90%, potentially leading to false-negative results in up to 20% of patients. The counter argument is that EUS-FNA may establish an atypical histologic diagnosis that may alter the surgical plans. Other types of malignancies, such as lymphoma or metastatic cancers to the pancreas, may mimic pancreatic adenocarcinoma and not require surgical intervention. In addition, patients (and some surgeons) may want to have a pathologic diagnosis prior to proceeding with a major surgery with considerable morbidity and mortality.
There have been at least 2 case reports of tumor seeding by EUS-FNA, although this is likely very rare.[22,23] This may pose a potential problem if the needle tract is not resected with the primary lesion, as in a distal pancreatic tumor. The decision to perform EUS-FNA of a resectable tumor is ultimately made after careful consultation between the endoscopist, patient, and surgeon.
Methods of EUS-FNA of Pancreatic Lesions
Performing EUS-FNA of pancreatic lesions is considered to be more difficult than performing EUS-FNA of lymph nodes and liver lesions. The fibrotic and inflammatory nature of many pancreatic tumors decreases the yield of FNA passes. One study found that an average of 5–6 separate FNAs of pancreatic lesions were necessary to achieve maximal accuracy as opposed to 2–3 for liver or lymph node targets.[24] The variation is quite wide, however. In our own experience, 5–6 passes on average are needed, although in rare cases more than 10 may be needed to obtain a definite diagnosis. The FNA needle size does not appear to have a significant impact on the yield of the FNA.[25] EUS-FNA of solid lesions has been demonstrated to be a very safe procedure. In fact, the number of needle passes was not predictive of complications.[26,27]
The use of trucut biopsy needles (TCB) has been evaluated in the diagnosis of pancreatic lesions. This large-caliber biopsy needle acquires a larger tissue sample with preserved tissue architecture that allows for histologic examination rather than simple cytologic review. A 19-gauge needle, with an 18-mm specimen tray is available for use through a linear echoendoscope. In a study comparing EUS-FNA with EUS-TCB, the EUS-TCB had an increased accuracy over EUS-FNA (85% vs 60%). However, this was not statistically significant.[28] Larghi and colleagues[29] evaluated the use of EUS-TCB in the diagnosis of pancreatic masses. EUS-TCB was successful in all cases in which the lesions were accessible from the stomach; however, when lesions were accessible only from the duodenum, EUS-TCB was successful in only 40% of cases. The overall accuracy of EUS-TCB was 61%, but when the calculation of accuracy was limited to those cases with a successful EUS-TCB and had long-term follow-up, the accuracy increased to 87%.[29] The transduodenal approach is technically difficult, because the scope needs to be as straight as possible to allow for effective deployment. Numerous anecdotal reports of endoscope damage by needle puncture have lessened the enthusiasm for EUS-TCB from the duodenum. The preliminary experience with EUS-TCB has shown that this is a safe and accurate method of procuring tissue if the pancreatic lesion is accessible from the stomach. EUS-TCB is not the recommended approach from the duodenum at this time.
Pancreatic Tumor Ablation
The ability of EUS to precisely localize and place needles into pancreatic tumors has sparked interest in pancreatic tumor ablation. Because most pancreatic cancer is not curable at the time of diagnosis, palliation of symptoms is the primary goal of therapy. The cause of morbidity in pancreatic cancer is largely due to mass effect and local invasion of the tumor, leading to pain, biliary obstruction, and duodenal obstruction. Chemotherapy is generally ineffective, and external beam radiation is associated with significant collateral damage to the adjacent organs. EUS-guided ablation of pancreatic tumors through local delivery of radiofrequency energy, brachytherapy, immunotherapy, or gene therapy is under investigation (Figure).[30–34]
Figure.
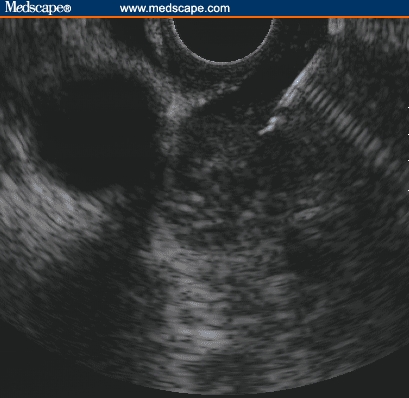
EUS-guided ablation of pancreatic tumor. FNA needle is placed in the mass. Various agents can be injected into the mass for ablation.
Conclusions
EUS-FNA has become an essential tool in the diagnosis and staging of pancreatic tumors. The major advantage of EUS-FNA lies in the ability of EUS to detect unresectable disease, to prevent unnecessary surgical exploration, and to diagnose small lesions undetectable by other imaging modalities. The future of EUS will be its expansion into therapeutic interventions, such as EUS-guided injection therapy for patients with unresectable pancreatic cancer.
Contributor Information
Kyung W. Noh, Division of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, Florida.
Michael B. Wallace, Division of Gastroenterology and Hepatology; Endoscopic Research, Mayo Clinic, Jacksonville, Florida.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer Statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Rocha Lima CM, Centeno B. Update on pancreatic cancer. Curr Opin Oncol. 2002;14:424–30. doi: 10.1097/00001622-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Demure MJ, Doffek KM, Komorowski RA, et al. Adenocarcinoma of the pancreas. Cancer. 1998;83:1328–1334. [PubMed] [Google Scholar]
- 4.Bret PM, Nicolet V, Labaie M. Percutaneous fine-needle aspiration biopsy of the pancreas. Diag Cytopathol. 1986;2:221–227. doi: 10.1002/dc.2840020309. [DOI] [PubMed] [Google Scholar]
- 5.Brandt KR, Charboneau JW, Stephens DH, et al. CT and US-guided biopsy of the pancreas. Radiology. 1993;187:99–104. doi: 10.1148/radiology.187.1.8451443. [DOI] [PubMed] [Google Scholar]
- 6.Lee JG, Leung J. Tissue sampling at ERCP in suspected pancreatic cancer. Gastrointest Endosc Clin N Am. 1998;8:221–235. [PubMed] [Google Scholar]
- 7.Raut CP, Grau AM, Staerkel GA, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118–128. doi: 10.1016/S1091-255X(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–2668. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 9.Harewood GC, Wiersema MJ. Endsonography-guided fine needle aspiration biospy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 10.Ylagan LR, Enmundowicz S, Kasal K, et al. Endoscopic ultrasound guided fine-needle aspiration cytology of pancreatic carcinoma. A 3-year experience and review of the literature. Cancer Cytopathol. 2002;96:362–369. doi: 10.1002/cncr.10759. [DOI] [PubMed] [Google Scholar]
- 11.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1996;45:387–393. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 12.Bhutani MS, Hawes RH, Baron PL, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854–858. doi: 10.1055/s-2007-1004321. [DOI] [PubMed] [Google Scholar]
- 13.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine-needle aspiration biopsy: A large single center experience. Gut. 1999;44:720–726. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binmoeller KF, Thul R, Rathod V, et al. Endoscopic ultrasound-guided, 18 gauge, fine needle aspiration of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121–127. doi: 10.1016/s0016-5107(98)70343-8. [DOI] [PubMed] [Google Scholar]
- 15.Gress FG, Hawes RH, Savides TJ, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsies using linear array and radial scanning endosonography. Gastrointest Endosc. 1999;45:243–250. doi: 10.1016/s0016-5107(97)70266-9. [DOI] [PubMed] [Google Scholar]
- 16.Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–249. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritscher-Ravens A, Izbicki J, Sri Ram PVJ, et al. Endosonography guided aspiration cytology extending the indication for organ-preserving pancreatic surgery. Am J Gastroenterol. 2000;95:2255–2260. doi: 10.1111/j.1572-0241.2000.02311.x. [DOI] [PubMed] [Google Scholar]
- 18.Afify AM, al-Khafaji BM, Kim B, et al. Endoscopic ultrasound-guided fine needle aspiration of the pancreas. Diagnostic utility and accuracy. Acta Cytol. 2003;47:341–348. doi: 10.1159/000326531. [DOI] [PubMed] [Google Scholar]
- 19.Fritscher-Ravens A, Brand L, Knofel T, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchymal and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–2775. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 20.tenBerge J, Hoffman BJ, Hawes RH, et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859–862. doi: 10.1067/mge.2002.124557. [DOI] [PubMed] [Google Scholar]
- 21.Tierney WM, Carpenter SL, Bansal R, et al. Accuracy and economic impact of helical CT and endoscopic ultrasound in the staging of ampullopancreatic tumors. Gastrointest Endosc. 1997;45:AB 183. [Google Scholar]
- 22.Paquin SC, Gariepy G, Lepanto L, et al. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610–611. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 23.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:609–605. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 24.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 25.Fritscher-Ravens A, Topalidis T, Bobrowski C, et al. Endoscopic ultrasound-guided fine-needle aspiration in focal pancreatic lesions: a prospective intraindividual comparison of two needle assemblies. Endoscopy. 2001;33:484–490. doi: 10.1055/s-2001-14970. [DOI] [PubMed] [Google Scholar]
- 26.Sahai AV, Schembre D, Stevens PD, et al. A multicenter U.S. experience with EUS-guided fine-needle aspiration using the Olympus GF-UM30P echoendoscope: safety and effectiveness. Gastrointest Endosc. 1999;50:792–796. doi: 10.1016/s0016-5107(99)70160-4. [DOI] [PubMed] [Google Scholar]
- 27.O'Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–474. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 28.Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 29.Larghi A, Verna EC, Stavropoulos SN, et al. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: A prospective study. Gastrointest Endosc. 2004;59:185–190. doi: 10.1016/s0016-5107(03)02538-0. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
- 31.Chang KJ, Nguyen PT, Thompson JA, et al. Phase I Clinical Trial of Allogeneic Mixed Lymphocyte Culture (Cytoimplant) Delivered by Endoscopic Ultrasound-Guided Fine Needle Injection in Patients with Advanced Pancreatic Carcinoma. Cancer. 2000;88:1325–1335. doi: 10.1002/(sici)1097-0142(20000315)88:6<1325::aid-cncr8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Hecht JR, Bedford R, Abbruzzese JL, et al. A Phase I/II Trial of Intratumoral Endoscopic Ultrasound Injection of ONYX-015 with Intravenous Gemcitabine in Unresecatable Pancreatic Carcinoma. Clin Can Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 33.Nori D, Merimsky O, Osin AD, et al. Palladium-103: A new radioactive source in the treatment of unresectable carcinoma of the pancreas. J Surg Oncol. 1996;61:300–305. doi: 10.1002/(SICI)1096-9098(199604)61:4<300::AID-JSO14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Wallace MB, Hawes RH. Emerging indications for EUS. Gastrointest Endosc. 2000;52:S55–S60. doi: 10.1067/mge.2000.110714. [DOI] [PubMed] [Google Scholar]


