Abstract and Introduction
Abstract
Aims
To compare the proportion of patients at high risk for coronary heart disease (CHD) achieving the recommended low-density lipoprotein cholesterol (LDL-C) treatment goal of < 100 mg/dL and the optional LDL-C target of < 70 mg/dL with coadministration of ezetimibe and simvastatin (EZE/SIMVA) vs either atorvastatin or simvastatin monotherapy.
Patients
Patients with established CHD or CHD risk equivalent according to National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria with baseline LDL-C ≥ 130 mg/dL and triglycerides (TG) ≤ 350 mg/dL.
Methods
A post hoc analysis from 2 separate studies assessed the percentage of high-risk patients achieving the LDL-C targets (< 100 and < 70 mg/dL) after 6 weeks on the usual recommended starting doses of the following treatments: EZE/SIMVA (10/20 mg) vs atorvastatin (10 mg) or simvastatin (20 mg). Depending on the study, EZE/SIMVA 10/10 or 10/40 mg was also compared with either atorvastatin 10 mg or simvastatin 20 mg. Percent change in other lipid parameters from baseline to study endpoint was also examined.
Results
In both studies, the proportions of patients achieving an LDL-C of < 100 mg/dL were significantly (P < .001) greater for EZE/SIMVA 10/10, 10/20, or 10/40 mg vs either atorvastatin 10 mg or simvastatin 20 mg after 6 weeks. The percentage reaching the optional LDL-C treatment target of < 70 mg/dL was also significantly higher with EZE/SIMVA compared with either atorvastatin or simvastatin. Percent reduction in LDL-C was significantly (P < .001) larger with all doses of EZE/SIMVA (46% to 59%) compared with either atorvastatin 10 mg (37%) or simvastatin 20 mg (38%) monotherapy after 6 weeks. Changes in other lipid parameters consistently favored EZE/SIMVA vs statin monotherapy. All treatments were well tolerated in both studies.
Conclusion
Patients at high risk for CHD are more likely to attain LDL-C treatment targets with the usual recommended starting dose of EZE/SIMVA (10 or 20 mg) therapy than with that of atorvastatin (10 mg) or simvastatin (20 mg) monotherapy.
Introduction
Despite the wealth of clinical trial data and treatment guidelines describing the clinical benefits of lipid-lowering treatments, many patients at high risk for CHD do not reach low-density lipoprotein cholesterol (LDL-C) treatment goal (<100 mg/dL) on lipid-lowering therapies.[1,2] Selection of therapies, inadequate efficacy of starting doses of lipid-lowering therapies, poor patient compliance, and patient/physician reluctance to titrate to higher doses of statins or other lipid-lowering drugs because of safety concerns are among the explanations for the shortfall in LDL-C goal attainment.[3–6] Furthermore, the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) recently recommended a lower and more aggressive LDL-C target of less than 70 mg/dL as a therapeutic option for patients at very high risk.[7] Thus, more effective lipid-lowering therapies are needed to reach the established LDL-C goal (< 100 mg/dL) and the new, optional LDL-C treatment target (< 70 mg/dL).
The combination tablet containing ezetimibe and simvastatin (EZE/SIMVA) is a cholesterol-lowering agent that affects cholesterol metabolism through two different mechanisms of action: inhibition of cholesterol absorption in the intestine and inhibition of cholesterol synthesis in the liver. Coadministration of EZE/SIMVA has been demonstrated to be more effective in lowering LDL-C and increasing the proportion of patients achieving NCEP-ATP III LDL-C treatment targets than comparable or higher doses of atorvastatin or simvastatin monotherapy.[8–12]
Therefore, the purpose of this analysis was to compare the proportion of patients at high risk for CHD achieving LDL-C targets on the usual recommended starting dose of the dual therapy of EZE/SIMVA vs that of atorvastatin or simvastatin monotherapy.
Methods
This post hoc analysis examined LDL-C goal achievement in high risk patients with data from 2 large clinical trials.[8,9] Both studies had an initial 6-week period during which subjects were randomly assigned to study treatments; thereafter, statin doses were increased over subsequent study periods. More detailed methods for these studies have been previously published.[8,9] Only data from the initial 6-week study period were utilized for this report. One study[8] enrolled patients across different CHD risk strata, including high risk patients, while the second study[9] recruited only patients with established CHD or CHD risk equivalent according to NCEP ATP III criteria. Both studies had identical lipid entry criteria for the high-risk patients (LDL-C ≥ 130 mg/dL and TG ≤ 350 mg/dL). One study randomized patients equally to 1 of the following 3 daily treatments for 6 weeks: atorvastatin 10 mg; EZE/SIMVA 10/10 mg; or EZE/SIMVA 10/20 mg.[8] The second study randomized subjects according to a 5:5:2:2 ratio to 1 of the following 4 daily treatments for 6 weeks: simvastatin 20 mg; EZE/SIMVA 10/10 mg; EZE/SIMVA 10/20 mg; or EZE/SIMVA 10/40 mg.[9]
Treatment LDL-C levels from these studies were used to compare the ability of usual recommended starting doses for atorvastatin 10 mg,[13] simvastatin 20 mg,[14] and EZE/SIMVA 10/20 mg[15] to attain the NCEP ATP III LDL-C goal of < 100 mg/dL in high-risk patients. Further, the ability of these treatments to reach the new NCEP ATP III LDL-C therapeutic option of < 70 mg/dL was also compared. Data for EZE/SIMVA 10/10 mg and 10/40 mg were also presented for completeness.
Statistical Analysis
The proportions of patients reaching the LDL-C treatment goal of < 100 mg/dL were compared among treatment groups within each study. Only patients with baseline LDL-C above the specific target were included in the analyses. Complete details of the statistical analyses for the two studies were published elsewhere.[8,9] The statistical models differed between studies, and thus no inferential testing was performed between studies. In Ballantyne and colleagues,[8] comparison of the percentage of patients attaining the LDL-C goal of < 100 mg/dL after 6 weeks of treatment with EZE/SIMVA 10/10 or 10/20 mg to that with atorvastatin 10 mg was done with a logistic regression model with terms for treatment, baseline LDL-C, and baseline LDL-C randomization strata (≥ 130-160 mg/dL, ≥ 160-190 mg/dL, ≥ 190 mg/dL). For Feldman and colleagues,[9] a logistic regression model with terms for treatment, baseline LDL-C, and CHD risk category ([1] CHD or CHD risk equivalents without diabetes; [2] CHD or CHD risk equivalents with type 2 diabetes; or [3] type 2 diabetes only] was used to compare the percentage of patients attaining the LDL-C goal of < 100 mg/dL after 6 weeks of treatment with EZE/SIMVA 10/10, 10/20, or 10/40 mg to that with simvastatin 20 mg. Odds ratio estimates derived from the logistic regression models and 95% confidence intervals (CI) were used to quantify treatment effect. Similar statistical models were used to assess the percentage of high-risk patients achieving the optional LDL-C target of < 70 mg/dL. Percent changes from baseline for the lipid parameters were analyzed with analysis of covariance models or, when needed, the nonparametric equivalent.
In the analysis, safety data were reported for the high-risk subgroup from Ballantyne and colleagues[8] and summarized for the previously reported data for Feldman and colleagues.[9] In contrast to the efficacy comparisons, which were based on the first 6-week treatment period for both studies in the present analysis, the safety and tolerability data presented were from the entire 24-week (four 6-week periods) active treatment phase. Moreover, all randomized, high-risk patients were included in the safety assessment.
Results
Baseline demographics and lipid profiles of the patients with CHD or CHD-risk equivalent including diabetes (ie, high risk) were generally well balanced within each study (Table 1). Mean baseline LDL-C ranged from 165 to 174 mg/dL across groups. In Ballantyne and colleagues,[8] high-risk patients represented approximately 43% of the entire cohort of patients across the CHD risk strata, whereas all patients in Feldman and colleagues[9] were enrolled because of their high-risk status.
Table 1.
Baseline Characteristics of Patients With CHD or CHD-Risk Equivalent
| Variables* | High-risk Subgroup From Ballantyne and Colleagues[8] | Feldman and Colleagues[9] | |||||
|---|---|---|---|---|---|---|---|
| Atorvastatin 10 mg n = 109 | EZE/SIMVA 10/10 mg n = 124 | EZE/SIMVA 10/20 mg n = 104 | Simvastatin 20 mg n = 253 | EZE/SIMVA 10/10 mg n =251 | EZE/SIMVA 10/20 mg n =109 | EZE/SIMVA 10/40 mg n = 97 | |
| Age (yr) | 65.0 (8.7) | 62.3 (9.8) | 63.9 (10.1) | 62.1 (9.7) | 61.3 (10.2) | 64.0 (9.8) | 61.7 (9.8) |
| BMI (kg/m2) | 30.2 (6.8) | 30.8 (6.3) | 29.7 (5.6) | 30.5 (6.3) | 31.4 (5.9) | 31.0 (5.7) | 31.2 (6.8) |
| Men (%) | 61.5 | 62.9 | 57.7 | 62.5 | 68.9 | 54.1 | 61.9 |
| Lipid Parameters (mg/dL) | |||||||
| LDL-C | 174.2 (42.7) | 172.2 (39.1) | 167.4 (34.3) | 173.8 (44.7) | 165.1 (34.3) | 167.3 (33.0) | 170.5 (40.6) |
| TC | 258.6 (47.2) | 255.8 (43.0) | 252.4 (36.4) | 256.7 (46.8) | 247.6 (38.0) | 248.8 (37.9) | 252.3 (43.7) |
| HDL-C | 44.5 (10.4) | 43.5 (11.2) | 45.7 (9.4) | 46.1 (11.2) | 44.6 (10.2) | 45.2 (10.6) | 46.5 (11.1) |
| TG† | 169.0 (90.2) | 180.0 (96.7) | 187.0 (113.5) | 169.5 (88.8) | 181.5 (100.9) | 177.0 (87.4) | 168.0 (89.3) |
| Non-HDL-C | 214.1 (47.5) | 212.3 (41.9) | 206.7 (36.3) | 210.7 (46.6) | 203.0 (37.7) | 203.6 (36.1) | 205.8 (42.3) |
| apo B | 166.6 (31.7) | 167.7 (32.6) | 161.8 (27.3) | 163.7 (34.0) | 159.7 (29.2) | 158.9 (29.0) | 162.5 (31.4) |
| apo A-I | 146.1 (24.8) | 143.6 (26.6) | 147.8 (20.9) | 150.4 (25.8) | 148.2 (25.2) | 148.3 (27.5) | 151.6 (25.6) |
Data are presented as mean ± standard deviation or frequency
Median (SD for median) EZE/SIMVA = ezetimibe/simvastatin; BMI = body mass index; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides; non-HDL-C = non-high-density lipoprotein cholesterol; apo = apolipoprotein
For the LDL-C goal of < 100 mg/dL, significantly more high-risk patients on the usual recommended starting dose of EZE/SIMVA 10/20 mg reached this goal compared with those on atorvastatin 10 mg or simvastatin 20 mg.
The results with the other doses of EZE/SIMVA were also significantly (P ≤ .001) greater when compared to atorvastatin or simvastatin monotherapy. For patients on EZE/SIMVA 10/10 mg in Ballantyne and colleagues,[8] 70% attained LDL-C < 100 mg/dL after the first treatment period. In Feldman and colleagues,[9] 75% and 88% of high-risk patients on EZE/SIMVA 10/10 mg and 10/40 mg, respectively, achieved an LDL-C of < 100 mg/dL after 6 weeks.
In both studies, the proportion of high-risk patients achieving the LDL-C treatment option of < 70 mg/dL was significantly (P ≤ .001) greater for EZE/SIMVA across the dose range studied vs either atorvastatin 10 mg or simvastatin 20 mg. Figure 2 presents the percentages of patients reaching LDL-C < 70 mg/dL at the usual recommended starting doses for each therapy.
Figure 2.
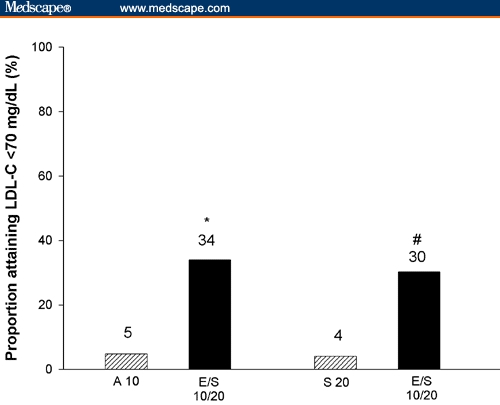
Proportion of high-risk patients achieving the LDL-C treatment option of < 70 mg/dL at week 6 (*P < .001 EZE/SIMVA 10/20 mg [E/S 10/20] vs atorvastatin 10 mg [A 10]; #P < .001 EZE/SIMVA 10/20 mg [E/S 10/20] vs simvastatin 10 mg [S 10]).
Additionally, more patients on EZE/SIMVA 10/10 mg lowered their LDL-C below 70 mg/dL compared with atorvastatin 10 mg (20% vs 5%, respectively, P ≤ .001). Attainment of the optional target of < 70 mg/dL was also significantly (P ≤ .001) greater with EZE/SIMVA 10/10 mg (25%) and 10/40 mg (59%) when compared with simvastatin 20 mg (5%).
Percent reduction in LDL-C was significantly (P ≤ .001) greater with all doses of EZE/SIMVA compared with either simvastatin or atorvastatin monotherapy (Table 2). Coadministration of EZE/SIMVA at all doses analyzed produced significantly greater reductions in total cholesterol (TC), apolipoprotein (apo) B, and non-HDL-C compared with both statin monotherapies. Higher doses of EZE/SIMVA (10/20 mg and 10/40 mg) produced significant changes in triglycerides (TG) and HDL-C relative to simvastatin monotherapy, but no differences between treatments were observed for apo A-I. By contrast, EZE/SIMVA 10/10 mg and 10/20 mg significantly improved TG, HDL-C, and apo A-I to a greater degree than did atorvastatin 10 mg.
Table 2.
Percent Change in Lipid Parameters From Baseline to the End of First Treatment Period
| Variables* | High-risk Subgroup From Ballantyne and Colleagues[8] | Feldman and Colleagues[9] | |||||
|---|---|---|---|---|---|---|---|
| Atorvastatin 10 mg | EZE/SIMVA 10/10 mg | EZE/SIMVA 10/20 mg | Simvastatin 20 mg | EZE/SIMVA 10/10 mg | EZE/SIMVA 10/20 mg | EZE/SIMVA 10/40 mg | |
| LDL-C | −37 (1.3) | −46 (1.2)‡ | −50 (1.3)‡ | −38 (0.8) | −47 (0.8)† | −53 (1.2)† | −59 (1.3)† |
| TC | −29 (0.9) | −34 (0.9)‡ | −36 (1.0)‡ | −27 (0.7) | −33 (0.6)† | −38 (0.9)† | −42 (1.0)† |
| HDL-C | 2.9 (1.2) | 8.2 (1.1)# | 8.7 (1.3)‡ | 5.1 (0.7) | 6.2 (0.7) | 8.0 (1.0)§ | 7.4 (1.1) |
| TG | −19 (2.8) | −27 (2.2)# | −29 (3.1)# | −19 (1.9) | −19 (1.5) | −25 (2.7)§ | −30 (2.6)† |
| Non-HDL-C | −35 (1.1) | −43 (1.0)‡ | −46 (1.1)‡ | −34 (0.8) | −42 (0.8)† | −48 (1.1)† | −53 (1.2)† |
| apo B | −32 (1.1) | −38 (1.1)‡ | −41 (1.2)‡ | −30 (0.8) | −36 (0.8)† | −41 (1.1)† | −47 (1.2)† |
| apo A-I | 0.0 (1.1) | 3.7 (1.1)* | 5.8 (1.2)‡ | 3.0 (0.8) | 3.0 (0.8) | 2.3 (1.1) | 1.2 (1.2) |
Data are presented as least square mean (SE) percent change from baseline to end of treatment period 1, except TG data are median (SE for median)
P ≤ .001 EZE/SIMVA 10/10 or 10/20 mg vs atorvastatin 10 mg
P ≤ .001 EZE/SIMVA 10/10, 10/20, or 10/40 mg vs simvastatin 20 mg
P ≤ .05 EZE/SIMVA 10/10 or 10/20 mg vs atorvastatin 10 mg
P ≤ .025 EZE/SIMVA 10/20 mg vs simvastatin 20 mg EZE/SIMVA = ezetimibe/simvastatin; LDL-C = low density lipoprotein cholesterol; TC = total cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides; non-HDL-C = non-high-density lipoprotein cholesterol; apo = apolipoprotein
In the high-risk subgroup from Ballantyne and colleagues,[8] the overall adverse event profile was comparable between EZE/SIMVA and atorvastatin monotherapy groups over the entire treatment period. There were no differences in the rates of discontinuations due to any reason among treatment groups. Consecutive elevations in hepatic transaminases ≥ 3 times upper limit of normal (ULN) were reported in 1 high-risk patient in the atorvastatin monotherapy group and 2 high-risk patients in the EZE/SIMVA 10/20 mg group. There were no cases of creatine phosphokinase (CPK) levels ≥ 10 times ULN found in the high-risk subgroup from Ballantyne and colleagues.[8] As previously reported in Feldman and colleagues[9] and briefly summarized herein, coadministration of EZE/SIMVA was well tolerated and had a safety profile comparable to that of simvastatin monotherapy over the 24-week treatment period. Treatment-related adverse events and discontinuations were similar across treatment groups. One patient in the EZE/SIMVA 10/10 mg and EZE/SIMVA 10/40 mg groups experienced consecutive elevations in hepatic transaminases ≥ 3 times ULN, but these patients completed the study. Elevated CPK levels of ≥ 10 times ULN were found in 3 patients: 2 in the simvastatin monotherapy group (1 with muscle symptoms) and 1 in the EZE/SIMVA 10/40 mg group (with muscle symptoms). Both cases in the simvastatin group were considered by the investigator to be related to exercise. The case in the EZE/SIMVA 10/40 mg group was considered by the investigator to be possibly related to treatment.[9]
Discussion
More high-risk patients (those with CHD or CHD risk equivalent including diabetes) reached the LDL-C treatment goal of < 100 mg/dL and the more aggressive treatment option of < 70 mg/dL with the usual recommended starting dose of EZE/SIMVA 10/20 mg compared with the usual starting doses of either atorvastatin (10 mg) or simvastatin (20 mg) within 6 weeks of initiating therapy. Among high-risk patients, 83% on EZE/SIMVA 10/20 mg were able to reach the established LDL-C goal of < 100 mg/dL compared with approximately 46% of those on either atorvastatin 10 mg or simvastatin 20 mg alone. The difference was more pronounced in the proportion achieving the new NCEP ATP III therapeutic option of LDL-C < 70 mg/dL. Approximately 5% of high-risk patients reached the aggressive therapeutic option of LDL-C < 70 mg/dL with atorvastatin 10 mg or simvastatin 20 mg monotherapy, whereas 30% to 34% of patients attained this target with EZE/SIMVA 10/20 mg. The proportion reaching LDL-C below 70 mg/dL was even greater (59%) with the alternative recommended starting dose of EZE/SIMVA (10/40 mg).
ATP III also advised that lipid-lowering therapies should produce at least a 30% to 40% reduction in LDL-C for high-risk or moderately high-risk patients.[7] Furthermore, lowering LDL-C by at least 45% has been demonstrated to slow or reverse the progression of carotid atherosclerosis.[16,17] In the present study, the usual recommended starting dose of atorvastatin (10 mg) and simvastatin (20 mg) resulted in at least a 37% reduction in LDL-C, whereas the recommended starting dose of EZE/SIMVA (10/20 mg) lowered LDL-C by 50% to 53%, and the alternative starting dose of EZE/SIMVA (10/40 mg) lowered LDL-C by 59%. Changes in other lipid parameters consistently favored EZE/SIMVA vs statin monotherapy. Safety and tolerability data demonstrated that EZE/SIMVA had a safety profile similar to that of either atorvastatin or simvastatin alone.[8,9]
The dose comparisons provided in this paper are particularly relevant because they are the recommended starting doses and most commonly used doses currently prescribed by physicians for EZE/SIMVA, simvastatin, and atorvastatin. EZE/SIMVA 10/20 mg represents 45% of all EZE/SIMVA prescriptions, while atorvastatin 10 mg is 46% of all atorvastatin prescriptions and simvastatin 20 mg is 41% of all simvastatin prescriptions (IMS NPA Retail – December 2004). Furthermore, since these results represent 6 weeks of treatment without dose titration, they provide a reasonable estimate of the LDL-C reductions that practicing physicians may expect at the first follow-up visit after initiating treatment. However, the current analyses may slightly underestimate goal attainment with these treatments in the high-risk population. Entry criteria in the trials required patients to have LDL-C levels ≥ 130 mg/dL,[8,9] requiring a ≥ 30 mg/dL reduction to reach the LDL-C goal of 100 mg/dL and a ≥ 60 mg/dL reduction to achieve the treatment option of 70 mg/dL. Therefore, it may be assumed that greater proportions of high-risk patients with LDL-C in the 100 to 130 mg/dL range would achieve the established and new targets based on the mean percent reductions in LDL-C noted with each treatment in the present study.
In conclusion, patients at high risk for CHD are more likely to attain standard and aggressive LDL-C treatment targets through the dual inhibitory actions of EZE/SIMVA therapy than with the usual recommended starting doses of atorvastatin or simvastatin monotherapy.
Figure 1.
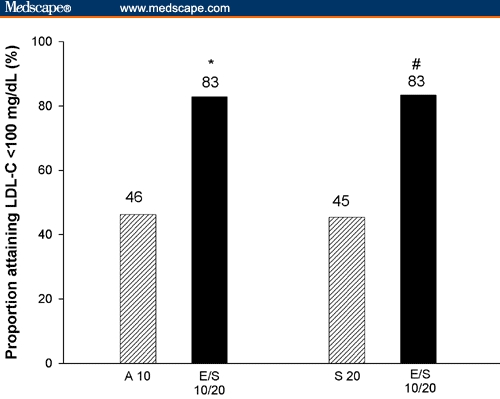
Proportion of high-risk patients achieving the recommended LDL-C treatment goal of < 100 mg/dL at week 6 (*P < .001 EZE/SIMVA 10/20 mg [E/S 10/20] vs atorvastatin 10 mg [A 10]; #P < 0.001 EZE/SIMVA 10/20 mg [E/S 10/20] vs simvastatin 10 mg [S 10]).
Funding Information
The studies, on which these analyses were based, were sponsored by Merck/Schering-Plough Pharmaceuticals, North Wales, Pennsylvania
Contributor Information
James McKenney, National Clinic Research, Inc., Richmond, Richmond, Virginia. Email: jmckenney@ncrinc.net.
Christie M. Ballantyne, Department of Medicine, Baylor College of Medicine; Center for Cardiovascular Disease Prevention, Methodist DeBakey Heart Center, Houston, Texas.
Theodore A. Feldman, Miami Research Associates, Coral Gables, Florida.
William E. Brady, Clinical Biostatistics, Merck & Co., West Point, Pennsylvania.
Arvind Shah, Clinical Biostatistics, Merck Research Laboratories, Rahway, New Jersey.
Michael J. Davies, Medical Communications, Merck Research Laboratories, Rahway, New Jersey.
Joanne Palmisano, Clinical Development, Merck & Co., West Point, Pennsylvania.
Yale B. Mitchel, Clinical Research, Merck Research Laboratories, Rahway, New Jersey.
References
- 1.Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med. 2000;160:459–467. doi: 10.1001/archinte.160.4.459. [DOI] [PubMed] [Google Scholar]
- 2.Pyorala K, Lehto S, De Bacquer D, et al. Risk factor management in diabetic and non-diabetic patients with coronary heart disease. Findings from the EUROASPIRE I AND II surveys. Diabetologia. 2004;47:1257–1265. doi: 10.1007/s00125-004-1438-z. [DOI] [PubMed] [Google Scholar]
- 3.Gaw A. A new reality: achieving cholesterol-lowering goals in clinical practice. Atheroscler Suppl. 2002;2:5–8. doi: 10.1016/s1567-5688(01)00018-6. [DOI] [PubMed] [Google Scholar]
- 4.Foley KA, Simpson RJ, Jr, Crouse JR, III, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol. 2003;92:79–81. doi: 10.1016/s0002-9149(03)00474-0. [DOI] [PubMed] [Google Scholar]
- 5.Frolkis JP, Pearce GL, Nambi V, et al. Statins do not meet expectations for lowering low-density lipoprotein cholesterol levels when used in clinical practice. Am J Med. 2002;113:625–629. doi: 10.1016/s0002-9343(02)01303-7. [DOI] [PubMed] [Google Scholar]
- 6.Neal RC, Jones PH. Lipid-lowering: can ezetimibe help close the treatment gap? Cleve Clin J Med. 2003;70:777–783. doi: 10.3949/ccjm.70.9.777. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne CM, Blazing MA, King TR, et al. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol. 2004;93:1487–1494. doi: 10.1016/j.amjcard.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 9.Feldman T, Koren M, Insull W, Jr, et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol. 2004;93:1481–1486. doi: 10.1016/j.amjcard.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg AC, Sapre A, Liu J, et al. Efficacy and safety of ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2004;79:620–629. doi: 10.4065/79.5.620. [DOI] [PubMed] [Google Scholar]
- 11.Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 13.Product Information. LIPITOR (atorvastatin calcium) Dublin, Ireland: Pfizer; August 2003. [Google Scholar]
- 14.Whitehouse Station, NJ: Merck & Co., Inc.; Product Information. ZOCOR (simvastatin) February 2004. [Google Scholar]
- 15.North Wales, Pennsylvania: Merck/Schering Plough Pharmaceuticals; Product Information. VYTORIN (ezetimibe/simvastatin) July 2004. [Google Scholar]
- 16.Smilde TJ, van Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357:577–581. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 17.Smilde TJ, van den Berkmortel FW, Wollersheim H, et al. The effect of cholesterol lowering on carotid and femoral artery wall stiffness and thickness in patients with familial hypercholesterolaemia. Eur J Clin Invest. 2000;30:473–480. doi: 10.1046/j.1365-2362.2000.00654.x. [DOI] [PubMed] [Google Scholar]


