Abstract
Estrogens and antiestrogens influence the G1 phase of the cell cycle. In MCF-7 breast cancer cells, estrogen stimulated cell cycle progression through loss of the kinase inhibitor proteins (KIPs) p27 and p21 and through G1 cyclin-dependent kinase (cdk) activation. Treatment with antiestrogen drugs, Tamoxifen or ICI 182780, caused cell cycle arrest, with up-regulation of both p21 and p27 levels, an increase in their binding to cyclin E–cdk2, and kinase inhibition. The requirement for these KIPs in the arrests induced by estradiol depletion or by antiestrogens was investigated with antisense. Antisense inhibition of p21 or p27 expression in estradiol-depleted or antiestrogenarrested MCF-7 led to abrogation of cell cycle arrest, with loss of cyclin E-associated KIPs, activation of cyclin E–cdk2, and S phase entrance. These data demonstrate that depletion of either p21 or p27 can mimic estrogen-stimulated cell cycle activation and indicate that both of these KIPs are critical mediators of the therapeutic effects of antiestrogens in breast cancer.
Estradiol is mitogenic in up to 50% of de novo breast cancers, causing recruitment of quiescent cells into G1 and shortening the G1-to-S phase interval (1, 2). Although 70% of breast cancers express the estrogen receptor (ER), only two-thirds of these will respond to antiestrogens, of which, Tamoxifen is the most widely used (3, 4). Antiestrogens, such as Tamoxifen, its active metabolite, 4-hydroxytamoxifen (4-OH TAM), and the more potent steroidal antiestrogen ICI 182780 (Faslodex) lead to a G0/G1 arrest in susceptible ER-positive breast cancer cells (5–8). Unfortunately, hormonally responsive breast cancers invariably develop resistance to antiestrogens despite the continued expression of wild-type ER in most cases (9–12). Estrogens induce conformational changes in the ER, which promote its nuclear localization, dimerization, and function as a ligand-activated transcription factor (13–15). In addition, ligand binding to the ER can rapidly and transiently activate signal transduction pathways, notably the mitogen-activated protein kinase in breast cancer and in other cell types (16, 17). Because antiestrogen resistance usually develops in the presence of an intact ER, the mechanisms by which ER modulates the cell cycle may be altered during breast cancer progression. The evolution of prostate cancer to hormone independence also occurs without loss of the androgen receptor (18, 19) and may reflect a common mechanism of cell cycle misregulation.
Progression through the cell cycle is governed by a family of cyclin-dependent kinases (cdks), whose activity is regulated by phosphorylation (20), activated by cyclin binding (21, 22), and inhibited by the cdk inhibitors of the inhibitor of cdk4 (INK4) family (p16INK4A, p15INK4B, p18, and p19) and kinase inhibitor protein (KIP) family (p21WAF-1/CIP-1, p27Kip1, and p57KIP2; refs. 22–24). Passage through G1 into S phase is regulated by the activities of cyclin D-, cyclin E-, and cyclin A-associated kinases. Although p27 protein is strongly expressed in normal mammary epithelial tissue, decreased levels of p27 protein in primary breast cancers are correlated with poor prognosis (25, 26) and steroid independence (25). Reduced p21 levels have also been associated with a poor prognosis in some breast cancer studies (27–29). Expression of the ER, a good prognostic factor in breast cancer, is associated with higher levels of both p21 and p27 proteins (25, 27, 28, 30). Our observation that loss of p27 was strongly associated with hormone independence (25) stimulated the present investigation of the role of these KIPs in cell cycle effects of estrogen and antiestrogens in breast cancer cells.
Although recent reports correlate estrogenic stimulation with activation of cyclin E–cdk2, some suggest the importance of the cdk inhibitor p21 (31, 32) and others emphasize a role for p27 (33). An understanding of how estrogens and antiestrogens influence the cell cycle and the mechanisms of their alteration in cancer progression may facilitate the development of new hormonal treatments for breast cancer and other hormone-dependent cancers. The present study provides evidence that both p21 and p27 play essential roles in the cell cycle arrest of breast cancer cells by antiestrogens.
Materials and Methods
Cell Culture and Synchronization.
MCF-7 cells (34) were grown in improved modified essential medium (IMEM-option Zn2+) supplemented with insulin and 5% (vol/vol) FCS. Cells were transferred to phenol red-free medium for 48 h and then synchronized in quiescence by depletion of estradiol through transfer to IMEM-option Zn2+ supplemented with 5% (vol/vol) charcoal-stripped FCS for 48 h.
Analysis of Cell Cycle Regulators.
Cells were released from quiescence by readdition of 10 nM 17-β-estradiol (estradiol) at t = 0 h. At intervals thereafter, cells were harvested for cell cycle analysis by flow cytometry after BrdUrd pulse labeling and propidium iodide counterstaining (35) and for Western analysis of cyclins E, D1, A, and B, as well as cdk2, cdk4, cdk6, p15, p21, and p27 proteins (from 20–50 μg of protein extract) as described (36). Equal protein loading was verified by probing these blots with antibody to β-actin (Sigma). Quantitation of proteins on Western and immunoprecipitation–Western blots was done by densitometry with imagequant software. Cyclin E was immunoprecipitated from 100–150 μg of protein lysate with anti-cyclin E mAb 172 and associated proteins detected by immunoblotting or associated kinase assayed with histone H1 as substrate as described (36). At the times indicated, Cdk4 immunoprecipitates (polyclonal antibody from Santa Cruz Biotechnology) were analyzed for associated proteins, and cyclin D1 was immunoprecipitated from 200 μg of protein with DCS-11 antibody (Neomarkers, Freemont, CA) for kinase assays with pRb substrate as described (37). Immunohistochemical analysis of p27 was undertaken at the indicated times after addition of estradiol as described (25).
Analysis of Cell Cycle Arrest by Inhibition of ER Signaling.
Asynchronous MCF-7 cultures were either depleted of estradiol as described above or arrested by addition of 1 μM 4-OH-TAM (Sigma) or 10 nM ICI 182780 {7α-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]estra-1,3,5(10),-triene-3,17β-diol; Zeneca Pharmaceuticals, Wilmington, DE} to complete medium, and samples were collected at 0, 12, 18, 24, and 48 h thereafter for protein and flow cytometric analyses. p21, p27, cyclins E, and D1 proteins were assayed by Western analysis, and cyclin E-associated cdk2, p21, p27, and histone H1 kinase activities were assayed as in ref. 36.
Antisense Oligonucleotide Transfections.
Phosphorothioate oligonucleotide sequences were as follows: GS5422 antisense p27 (ASp27), 5′-TGGCTCTCXTGCGCC-3′; GS5585 mismatch p27 (MSMp27), 5′-TGGCTCXCTTGCGCC-3′; GS6008 antisense p21 (ASp21), 5′-TCCGXGCCCAGCTCC-3′; GS6074 mismatch p21 (MSMp21), 5′-TCCGXCGCCAGCTCC-3′. X indicates the G clamp modification of these oligonucleotides (38). MCF-7 cells rendered quiescent by estradiol depletion or by treatment with 10 nM ICI 182780 or 1 μM 4-OH TAM were transfected with 120 nM oligonucleotides by using 2.5 μg/ml cytofectin G3815 (Gilead Scientific, Foster City, CA) for 6 h as described (38, 39), followed by replacement with complete medium. Flow cytometry and protein analysis were performed at 1, 21, and 28 h thereafter. Neither p21 nor p27 was increased in mismatch controls compared with lipid-only transfections.
Metabolic Labeling.
Before and at 1 h and 21 h after completion of antisense oligonucleotide transfection, cells were metabolically labeled, followed by immunoprecipitation of p21 and p27. Cells were incubated for 30 min in 8 ml of α-MEM lacking methionine and then labeled metabolically for 1 h with 500 μCi [35S]methionine in 2 ml of α-MEM lacking methionine. Cells were lysed on ice, clarified by centrifugation, and precleared with 1 μg of nonspecific rabbit polyclonal IgG and protein A Sepharose beads. [35S]Methionine incorporation was quantitated as trichloroacetic acid-insoluble counts. Lysates volumes representing equal amounts (108 cpm) of total trichloroacetic acid-incorporated counts were immunoprecipitated for 1 h with either p21 or p27 antibody or with control nonspecific rabbit polyclonal IgG. Immune complexes were collected on protein A Sepharose beads, washed three times, and eluted into Laemmli buffer. Samples were resolved by SDS/PAGE; gels were dried; and labeled proteins were visualized by autoradiography.
Results and Discussion
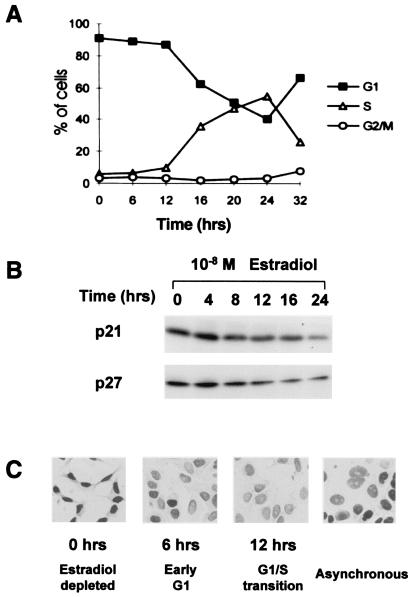
Estradiol stimulates a shift of KIP proteins from cyclin E–cdk2 into cyclin D1–cdk4. Estradiol stimulation of steroid-deprived, quiescent MCF-7 breast cancer cells induced synchronous cell cycle reentry. S phase entrance was detected by 12 h, with the peak percentage of S phase cells at 24 h (Fig. 1A). The levels of cyclin E, cdk2, and cdk4 remained constant, and p15INK4B protein levels fell as cells moved into G1 (data not shown). MCF-7 cells do not express p16INK4A because of deletion of this gene (40). Cyclin D1 was not detected in quiescent cells but rose within 3 h of estradiol addition and remained constant thereafter. p21 and p27 protein levels fell by 3- and 5-fold, respectively, by 24 h (Fig. 1B). Immunohistochemical analysis of p27 supported the immunoblotting data (Fig. 1C). Estradiol-depleted, quiescent MCF-7 cells showed strong nuclear p27 staining that was notably reduced by 6 h after addition of estradiol and barely detectable above background by 12 h as S phase entrance began.
Figure 1.
Losses of p21 and p27 during estradiol stimulation of quiescent MCF-7 cells. Quiescent, estradiol-depleted MCF-7 cells were stimulated by readdition of 10 nM estradiol, and samples were taken at intervals thereafter. (A) Cell cycle synchrony was determined by dual BrdUrd/propidium iodide pulse labeling and flow cytometric analysis. (B) p21 and p27 immunoblots revealed levels of these proteins during cell cycle progression. (C) p27 protein was assayed by immunohistochemistry in asynchronous cultures at the indicated times after estradiol stimulation of quiescent MCF-7 as described (25).
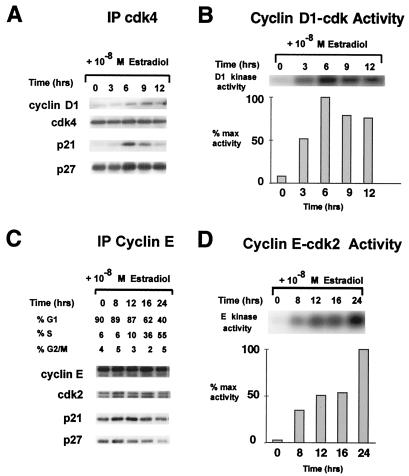
The pattern of binding of p21 and p27 to cdk4 and cdk2 complexes differed during estradiol-stimulated cell cycle progression in MCF-7. Cdk4-bound p27 was abundant in estradiol-depleted cells and increased in parallel with the increased assembly and activation of cyclin D1–cdk4 between 3 and 9 h after estradiol addition (Fig. 2 A and B). Cyclin D1-dependent kinase activities and cyclin D1 binding to cdk4 were reduced by 12 h and undetectable by 16 h. Although p21 protein was elevated, very little p21 was detectable in cdk4 complexes in quiescent MCF-7. As for p27, cyclin D1–cdk4 assembly was accompanied by increased p21 binding, in keeping with the function of p21 and p27 as positive regulators of cyclin D1–cdk4 assembly (37, 41). Although cyclin D2 and cdk6 are detectable in MCF-7, cyclin D1–cdk4 has been shown to be the major D-type cdk in these cells (31, 32). In contrast to their pattern in cyclin D1 complexes, activation of cyclin E–cdk2 after estradiol stimulation was correlated with loss of p27 and p21 from cyclin E–cdk2. Cyclin E–cdk2 activation was correlated with S phase entrance (Fig. 2 B and C).
Figure 2.
Different patterns of KIP binding during cyclin D1–cdk4 and cyclin E–cdk2 activation. Cdk4 (A) and cyclin E (C) immunoprecipitates (IP) from cell lysates recovered at intervals after readdition of estradiol to steroid-depleted MCF-7 cells were resolved and analyzed by immunoblotting with the indicated antibodies. Cyclin D1 (B) and cyclin E (D) immunoprecipitates were assayed for kinase activity (36) at intervals after estradiol stimulation of quiescent MCF-7.
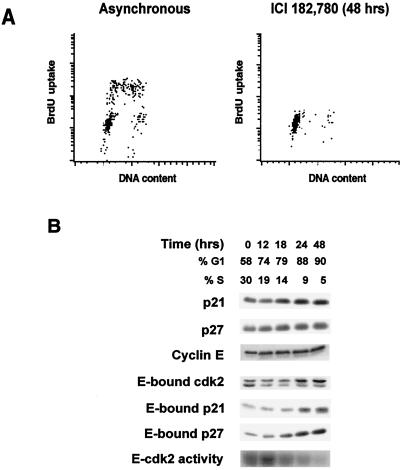
p21 and p27 Bind and Inhibit Cyclin E–cdk2 on Interruption of Mitogenic ER Signaling.
To investigate further cell cycle regulation by estrogen, ER signaling was interrupted in asynchronous MCF-7 cultures in three ways: by treatment with the pure ER antagonist ICI 182780, by the addition of antiestrogen 4-OH TAM, or by steroid depletion. All induced quiescence, with the S phase fraction falling from 29–36% to 1–5% over 48 h with a corresponding increase in the percentage of cells in G0/G1 phase (data shown for ICI 182780 in Fig. 3). p21 and p27 proteins increased, as did their binding to cyclin E–cdk2, in parallel with kinase inhibition (Fig. 3B). Although levels of cyclin E-bound cdk2 were unchanged, there was an accumulation of the slower-mobility, non-CAK-activated cdk2, lacking Thr-160 phosphorylation. The pattern of increase in p21 and p27 and of their binding to cyclin E during estradiol depletion and 4-OH TAM arrest were similar to that shown for ICI 182780 in Fig. 3B. The loss of cyclin D1 observed during antiestrogen treatment would lead to dissociation of KIPs from cyclin D1 complexes and foster KIP inhibition of cyclin E–cdk2 (42, 43).
Figure 3.
p21 and p27 proteins increase during G0/G1 arrest by ER blockade. Asynchronously growing MCF-7 cells were treated with the ER-blocking drug ICI 182780 (Faslodex) at time 0 h, and samples were collected for flow cytometry or protein analysis at times indicated. (A) Cell cycle distribution before and 48 h after drug treatment. (B) Lysates were analyzed by immunoblotting with the indicated antibodies. Cyclin E immunoprecipitates were immunoblotted for associated p21 or p27 or analyzed for associated histone H1 kinase activity as described in ref. 36. Similar results were obtained for arrests with 4-OH TAM or after transfer to estradiol-depleted, charcoal-stripped serum.
These data and earlier work support the notion that estrogens and antiestrogens work through changes in p21 and p27 levels. Foster and Wimalasen (33) showed that p27 immunoprecipitation significantly depleted cyclin E–cdk2 inhibitory activity from serum and amino acid-starved MCF-7 cells. Others showed that most of the cyclin E–cdk2 inhibitory activity in Tamoxifen- or ICI 182780-arrested MCF-7 was removed by immunodepletion of p21. However, immunodepletion of both p21 and p27 was required to deplete fully cyclin E from arrested cells, indicating that cyclin E is bound to either p21 or p27 in an ER-blocked arrest state (31, 32, 42). These authors proposed that the estradiol-stimulated up-regulation of cyclin D1 served to sequester p21 away from cyclin E complexes leading to activation of cyclin E and pRb phosphorylation. However, none of these earlier studies definitively established a requirement for p21 and p27 in cell cycle arrest by antiestrogens.
p21 and p27 Are Essential Mediators of Arrest by Antiestrogens.
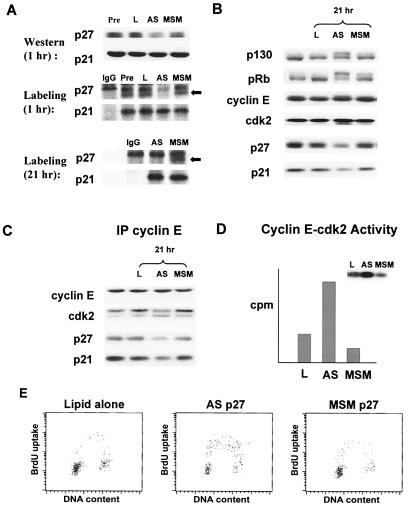
To test directly whether p21 and p27 play essential roles in the cell cycle arrest after blockade of ER signaling in MCF-7 cells, we used antisense oligonucleotides to inhibit p21 (ASp21) or p27 (ASp27) expression in cells arrested by ICI 182780, 4-OH TAM, or estradiol depletion (data shown for ASp27 in estradiol-depleted MCF-7 in Fig. 4). A G clamp heterocycle modification, a cytosine analog that clamps onto a guanine, was designed to enhance antisense/RNA interaction and showed increased antisense oligonucleotide potency over the C5 propynyl-modified oligonucleotides used in earlier assays (38, 39). Within 1 h of transfection, ASp27-treated cells showed a 4-fold reduction in p27 but no loss of p21 (Fig. 4A), and p27 levels reached a nadir at about 6 h after transfection. The ASp21 oligos showed a similar specificity with no immediate loss of p27. Metabolic pulse labeling of ASp27-transfected cells showed specific inhibition of p27 synthesis but no effect on p21 protein synthesis at 1 and 21 h after completion of the transfection (Fig. 4A).
Figure 4.
Requirement of p27 for cell cycle arrest by estradiol depletion. (A) Estradiol-depleted MCF-7 cells were lysed before (left lane) or 1 h after exposure to lipid only (L), ASp27 (AS), or MSMp27 (MSM) and were immunoblotted for p21 and p27. Before and at 1 and 21 h after ASp27 transfection, cells were metabolically pulse labeled with [35S]methionine, and p21 and p27 were immunoprecipitated from lysates containing equal trichloroacetic acid incorporation. The positions of metabolically labeled p27 are indicated by arrows. A nonspecific band, migrating close to p27 was present in all lanes, including the control nonspecific IgG lane. (B) Immunoblotting shows cell cycle regulatory protein levels before (left lane) or 21 h after transfection of lipid alone (L), ASp27, or MSMp27. Cyclin E immunoprecipitates (IP) were recovered from the same lysates as in B above and immunoblotted to detect associated proteins (C) or assayed for cyclin E-associated histone H1 kinase activity (D). (E) Flow cytometry 21 h after transfection with ASp27, lipid alone, or MSMp27.
Treatment with ASp27 led to hyperphosphorylation of pRb and p130, CAK phosphorylation at Thr-160 on cdk2 (Fig. 4B), loss of p27 binding to cyclin E–cdk2, and cyclin E–cdk2 activation (Fig. 4 C and D), all consistent with stimulation of G1-to-S phase progression. Similar findings were observed for ASp21-treated cells. ASp21 treatment of cells arrested by estradiol-depletion, 4-OH TAM, or ICI 182780 showed loss of p21 and loss of p21 from cyclin E–cdk2 with activation of this kinase accompanying S phase entry (not shown). These effects were not observed in the mismatch and lipid control groups. Results shown are representative of up to three repeat experiments. It is notable that ASp27-treated cells showed late down-regulation of p21 at 21 h after transfection. Metabolic labeling of ASp27-transfected cells showed persistent specific inhibition of p27 synthesis at 21 h but no inhibition of p21 synthesis as cells were entering S phase. Thus, the reduction of p21 protein is not due to inhibition of p21 synthesis by the ASp27 oligo. Rather, the ASp27-induced inhibition of p27 synthesis was sufficient to move cells out of quiescence, and the subsequent loss of p21 most likely reflects the changes in posttranslational regulation of p21 leading to its degradation at the G1-to-S phase transition (44). Data in Fig. 4 A–C for ASp27 treatment of ICI 182780-arrested cells were similar to results for ASp27 treatment of cells arrested by estradiol depletion or 4-OH TAM (not shown). Despite the continued blockade of ER signaling, ASp21 or ASp27 transfection stimulated cell cycle entry of cells arrested by steroid depletion, 4-OH TAM, or ICI 182780. The S phase fraction rose to 21–26% at 21 h (or 15 h after reaching minimum levels of the AS-targeted KIP protein, Figs. 4E and 5) and 29–36% at 28 h after transfection. Thus, ASp21 or ASp27 was sufficient to mimic the effect of estradiol on G1-to-S phase progression in MCF-7 cells. These data indicate that a key effect of ER signaling is to relieve KIP-mediated inhibition of cdk2.
Figure 5.
Requirement for p21 and p27 in G1 arrest by ER-blocking drugs or estradiol-depletion. MCF-7 cells were arrested by estradiol depletion or by treatment with 4-OH TAM or ICI 182780. The graph indicates the percentage of S phase cells after a 21-h exposure to lipid only (black bars), antisense (white bars), or mismatch (hatched bars) oligonucleotides to either p21 or p27.
ASp27 not only inhibits p27 synthesis but would also lead to an increase in p27 degradation. As cyclin E–cdk2 is liberated from bound p27, it then phosphorylates remaining p27 molecules on Thr-187, thereby accelerating p27 degradation (45–47). Moreover, the activation of cyclin E–cdk2 is autocatalytic through activation of Cdc25A by cyclin E–cdk2 (48, 49). Thus, a relatively small initial reduction in p27 can stimulate a significant loss of p27 from cyclin E complexes.
Although breast cancer cells arrested by interruption of ER signaling have high levels of both p21 and p27, only p27 is elevated in serum-starved fibroblasts, and p27 is essential for arrest by serum starvation in immortalized fibroblasts (50, 51). Although p21 can compensate in part for the lack of p27 in serum-starved p27-null murine embryo fibroblasts (52), the mechanisms of quiescence induced by serum starvation in fibroblasts and those induced by steroid depletion in malignant breast epithelial cells differ importantly. Our data demonstrate that the cell cycle arrest induced by estradiol depletion or ER blockade requires both p21 and p27 and that these KIPs are not merely up-regulated as a consequence of cell cycle arrest.
Mitogenic effects of estradiol include the up-regulation of cyclin D1 through increased transcription (42, 43) and stabilization of cyclin D1 protein by assembly with cdk4 and cdk6 (53, 54), the latter mediated by p21 and p27 (37, 41). In addition, the present data establish definitively that estradiol-mediated losses of p21 and p27 relieve the inhibition of cyclin E–cdk2 (31, 32, 42).
In estradiol-depleted MCF-7, although p21 and p27 were abundant, they were not competent to stabilize cyclin D1 via assembly with its cdk partners. Cyclin D1 synthesis is detectable in metabolically labeled quiescent breast epithelial cells, but its association with cdk4 or cdk6 is detectable only several hours after mitogenic stimulation (ref. 36 and S. Cariou and J. Slingerland, unpublished results). Thus, an important effect of estradiol may be the conversion of p21 and p27 from a form that does not support assembly of cyclin D–cdk complexes in G0, to one that does. Similarly, in serum-starved fibroblasts, p27 did not support cyclin D1–cdk4 complex formation even after ectopic cyclin D1 overexpression (55). Moreover, in p21/p27 null cells, overexpression of cyclin D1 did not permit its assembly with cdk4 (41). After estradiol stimulation in MCF-7, KIP-cyclin D1–cdk4 assembly occurred at 6–9 h, whereas the loss of p27 and p21 from cyclin E complexes was notable only somewhat later, after the time of peak sequestration of these KIPs in cyclin D1 complexes. Although induced overexpression of cyclin D1 can abrogate antiestrogen arrest (42, 43), the physiologic up-regulation of cyclin D1 stimulated by estradiol in MCF-7 may be insufficient, on its own, to mediate the shifts of the KIPs out of cdk2.
The ubiquitin-mediated degradation of p27 (56–59) requires its phosphorylation on Thr-187 (45–47). Degradation of p21 is also proteasome dependent but may differ importantly from that of p27 (44). Although cyclin E–cdk2 acts in vitro and in vivo to phosphorylate p27 on Thr-187, other kinases may act on p27 before its degradation. The transition of p21 and p27 from potent inhibitors of cyclin E–cdk2 in G0 to cyclin D-dependent kinase assembly factors may require phosphorylation early in G1, before cyclin E–cdk2 activation. We have observed an increase in p27 phosphorylation before the reduction in its steady-state levels in estradiol-stimulated MCF-7 (S. Cariou and J. Slingerland, unpublished results). Mitogen-activated protein kinase activation has been implicated in p27 degradation (60, 61). It will be of interest to determine whether the estradiol-dependent activation of mitogen-activated protein kinase (16, 17) or other mitogenic kinase pathways regulate the transition of p21 and p27 from high-affinity inhibitors of cyclin E–cdk2, to activators of cyclin D1–cdk assembly.
The approximation that over 4 million women with breast cancer are on Tamoxifen worldwide is a minimal estimate (refs. 3 and 4 and V. C. Jordan, personal communication). An increasing body of in vitro data and metaanalysis of large patient cohorts have confirmed the requirement for ER expression for the therapeutic efficacy of Tamoxifen (3, 4). The present study suggests that, in addition to the ER, a breast cancer cell must express functional p21 and p27 for Tamoxifen or Faslodex (ICI 182780) to mediate cytostatic effects. These observations raise the hypothesis that deregulation and loss of these KIPs may underlie the clinical phenomena of hormone independence and antiestrogen resistance in breast cancer.
Acknowledgments
We thank members of the Slingerland lab for helpful suggestions and critical review of the manuscript. This work was supported by grants from the U.S. Army Breast Cancer Research Program and the Burroughs Wellcome Fund to J.M.S., by an Ontario Graduate Studies Award to J.C.H.D., and by Medical Research Council funding to A.M.
Abbreviations
- cdk
cyclin-dependent kinase
- KIP
kinase inhibitor protein
- 4-OH TAM
4-hydroxytamoxifen
- INK4
inhibitor of cdk4
- ER
estrogen receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160016897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160016897
References
- 1.Musgrove E A, Sutherland R L. Cancer Biol. 1994;5:381–389. [PubMed] [Google Scholar]
- 2.Henderson B E, Roos R, Bernstein L. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- 3.Jordan V C. Breast Cancer Res Treat. 1995;36:267–285. doi: 10.1007/BF00713399. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 5.Sutherland R L, Green M D, Hall R E, Reddel R R, Taylor I W. Eur J Cancer Clin Oncol. 1983;19:615–621. doi: 10.1016/0277-5379(83)90177-3. [DOI] [PubMed] [Google Scholar]
- 6.Osborne C K, Boldt D H, Clark G M, Trent J M. Cancer Res. 1983;43:3583–3585. [PubMed] [Google Scholar]
- 7.Watts C K W, Brady A, Sarcevic B, deFazio A, Sutherland R L. Mol Endocrinol. 1996;9:1804–1813. doi: 10.1210/mend.9.12.8614416. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson R I, Francis A B, McClelland R A, Manning D L, Gee J M W. Endocr Relat Cancer. 1994;3:1–13. [Google Scholar]
- 9.Encarnacion C A, Ciocca D R, McGuire W L, Clark G M, Fuqua S A, Osborne C K. Breast Cancer Res Treat. 1993;26:237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- 10.Robertson J F R. Br J Cancer. 1996;73:5–12. doi: 10.1038/bjc.1996.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howell A, DeFriend D, Robertson J, Blamey R, Walton P. Lancet. 1995;245:29–30. doi: 10.1016/s0140-6736(95)91156-1. [DOI] [PubMed] [Google Scholar]
- 12.Howell A, DeFriend D J, Robertson J F R, Blamey R W, Anderson L, Anderson E, Sutcliffe F A, Walton P. Br J Cancer. 1996;74:300–308. doi: 10.1038/bjc.1996.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlmann T, Evans R M. Cell. 1997;90:391–397. doi: 10.1016/s0092-8674(00)80498-5. [DOI] [PubMed] [Google Scholar]
- 14.Beato M, Chavez S, Truss M. Steroids. 1996;61:240–251. doi: 10.1016/0039-128x(96)00030-x. [DOI] [PubMed] [Google Scholar]
- 15.Katzenellenbogen J A, O'Malley B W, Katzenellenbogen B S. Mol Endocrinol. 1996;10:119–131. doi: 10.1210/mend.10.2.8825552. [DOI] [PubMed] [Google Scholar]
- 16.Migliaccio A, DiDomenico M, Castona C, DeFalco A, Bontempo P, Nola E, Auricchio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 17.Collins P, Webb C. Nat Med. 1999;5:1130–1131. doi: 10.1038/13453. [DOI] [PubMed] [Google Scholar]
- 18.van der Kwast T H, Schalken J, Ruizeveld de Winter J A, van Vroonhoven C C, Mulder E, Boersma W, Trapman J. Int J Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 19.Ruizeveld de Winter J A, Janssen P J, Sleddens H M, Verleun-Mooijman M C, Trapman J, Brinkmann A O, Santerse A B, Schroder F H, van der Kwast T H. Am J Pathol. 1994;144:735–746. [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon M J, Kaldis P. Results Probl Cell Differ. 1998;22:79–109. doi: 10.1007/978-3-540-69686-5_4. [DOI] [PubMed] [Google Scholar]
- 21.Morgan D O. Nature (London) 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 22.Sherr C J. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 23.Reed S I, Bailly E, Dulic V, Hengst L, Resnitzky D, Slingerland J. J Cell Sci Suppl. 1994;18:69–73. doi: 10.1242/jcs.1994.supplement_18.10. [DOI] [PubMed] [Google Scholar]
- 24.Sherr C J, Roberts J M. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 25.Catzavelos C, Bhattacharya N, Ung Y C, Wilson J A, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, et al. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 26.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M. Cancer Res. 1997;57:1259–1263. [PubMed] [Google Scholar]
- 27.Wakasugi E, Kobayashi T, Tamaki Y, Ito Y, Miyashiro I, Komoike Y, Takeda T, Shin E, Takatsuka Y, Kikkawa N, et al. Am J Clin Pathol. 1997;107:684–691. doi: 10.1093/ajcp/107.6.684. [DOI] [PubMed] [Google Scholar]
- 28.Jiang M, Shao Z-M, Wu J, Lu J-S, Yu L-M, Yuan J-D, Han Q-X, Shen Z-Z, Fontana J A. Int J Cancer. 1997;74:529–534. doi: 10.1002/(sici)1097-0215(19971021)74:5<529::aid-ijc9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Tsihlias J, Kapusta L, Slingerland J. Annu Rev Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- 30.Saez A, Sanchez E, Sanchez-Beato M, Cruz M A, Chacon I, Munoz E, Camacho F I, Martinez-Montero J C, Mollejo M, Garcia J F, et al. Br J Cancer. 1999;80:1427–1434. doi: 10.1038/sj.bjc.6690539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prall O W J, Sarcevic B, Musgrove E A, Watts C K W, Sutherland R L. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 32.Planas-Silva M D, Weinberg R A. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster J, Wimalasen J. Mol Endocrinol. 1996;10:488–496. doi: 10.1210/mend.10.5.8732680. [DOI] [PubMed] [Google Scholar]
- 34.Soule H D, Vazquez J, Long A, Albert S, Brenan S. J Natl Cancer Inst. 1973;51:1409–1413. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 35.Petrocelli T, Poon R, Drucker D, Slingerland J, Rosen C. Oncogene. 1996;12:1387–1396. [PubMed] [Google Scholar]
- 36.Sandhu C, Garbe J, Daksis J, Pan C-H, Bhattacharya N, Yaswen P, Koh J, Slingerland J, Stampfer M R. Mol Cell Biol. 1997;17:2458–2467. doi: 10.1128/mcb.17.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaBaer J, Garrret M, Steenson M, Slingerland J, Sandhu C, Chou H, Fattaey A, Harlow H. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 38.Flanagan W M, Wolf J J, Olson P, Grant D, Lin K Y, Wagner R W, Matteucci M D. Proc Natl Acad Sci USA. 1999;96:3513–3518. doi: 10.1073/pnas.96.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.St. Croix B, Florenes V, Rak A, Flanagan J W, Bhattacharya N, Slingerland J M, Kerbel R S. Nat Med. 1996;2:1204–1210. doi: 10.1038/nm1196-1204. [DOI] [PubMed] [Google Scholar]
- 40.Musgrove E, Lilischkis R, Cornish A L, Lee S L, Setlur V, Seshari R, Sutherland R L. Int J Cancer. 1995;63:584–591. doi: 10.1002/ijc.2910630420. [DOI] [PubMed] [Google Scholar]
- 41.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watts C K, Sweeney K J E, Warlters A, Musgrove E A, Sutherland R L. Breast Cancer Res Treat. 1994;31:95–105. doi: 10.1007/BF00689680. [DOI] [PubMed] [Google Scholar]
- 43.Prall O W J, Rogan E M, Musgrove E A, Watts C K W, Sutherland R L. Mol Cell Biol. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheaff R J, Singer J D, Swanger J, Smitherman M, Roberts M J, Clurman B E. Mol Cell. 2000;5:403–410. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- 45.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 46.Montagnoli A, Fiore F, Eytan E, Carrano A C, Draetta G F, Hershko A, Pagano M. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlach J, Hennecke S, Amati B. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H, Okayama H. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann I, Draetta G, Karsenti E. EMBO J. 1994;13:4302–4310. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivard N, L'Allemain G, Bartek J, Pouyssegur J. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- 51.Coats S, Flanagan M, Nourse J, Roberts J M. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 52.Coats S, White P, Fero M L, Lacy S, Chung G, Randel E, Firpo E, Roberts J M. Curr Biol. 1999;9:163–173. doi: 10.1016/s0960-9822(99)80086-4. [DOI] [PubMed] [Google Scholar]
- 53.Bates S, Parry D, Bonetta L, Vousden K, Dickson C, Peters G. Oncogene. 1994;9:1633–1640. [PubMed] [Google Scholar]
- 54.Parry D, Bates S, Mann D J, Peters G. EMBO J. 1995;14:503–511. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C A, Kato J-Y. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 57.Tsvetkov L M, Yeh K H, Lee S J, Sun H, Zhang H. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 58.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 59.Carrano A, Eytan E, Hershko A, Pagano M. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 60.Alessandrini A, Chiaur D S, Erikson R, Pagano M. Leukemia. 1997;11:342–345. doi: 10.1038/sj.leu.2400581. [DOI] [PubMed] [Google Scholar]
- 61.Kawada M, Yamagoe S, Murakami Y, Suzuki K, Mizuno S, Uehara Y. Oncogene. 1997;15:629–637. doi: 10.1038/sj.onc.1201228. [DOI] [PubMed] [Google Scholar]