Abstract
Context
Chronic obstructive pulmonary disease (COPD) is recognized as a major healthcare problem in the United States and around the world.
Objective
This survey regarding initial experience in patients with COPD collected feedback about newly initiated therapy with fluticasone propionate/salmeterol (FSC; ADVAIR DISKUS).
Design
Three telephone surveys were conducted; Survey 1 prior to initiating therapy with FSC 250/50, and Surveys 2 and 3 at 2 weeks and 30 days after initiating therapy with FSC 250/50, respectively.
Setting
One thousand primary care physicians recruited outpatients into the trial.
Patients
Patients were either newly diagnosed with COPD associated with chronic bronchitis or were still experiencing breathing difficulties on an anticholinergic medication.
Interventions
Patients initiated FSC 250/50 and received a 1-month supply of FSC 250/50 with an albuterol inhaler for rescue use.
Main Outcome Measures
Outcome measures were patient perceptions of satisfaction, compliance, and convenience and changes in breathing on 1 (negative) to 9 (positive) point scales.
Results
Five hundred sixteen patients completed all 3 surveys. The mean age was 61 years, 63% were female, and 62% had been diagnosed with COPD associated with chronic bronchitis for 3 years or less (Table 1).
Conclusion
Patients reported high satisfaction, compliance, and convenience with FSC 250/50 within 2 weeks of initiating therapy, all maintained over the trial period. Additionally, patients had positive changes in breathing, including improvements in the ability to breathe upon awakening in the morning.
Readers are encouraged to respond to George Lundberg, MD, Editor of MedGenMed, for the editor's eye only or for possible publication via email: glundberg@medscape.net
Introduction
COPD is increasingly recognized as a major healthcare problem in the United States and around the world. The prevalence of COPD continues to grow, and its incidence is expected to rise during the coming decade.[1] However, the underdiagnosis of COPD is evidenced by the fact that only 10.5 million patients are diagnosed with the disease while 24 million people have evidence of airflow obstruction.[2]
While, historically, healthcare practitioners have felt that limited treatment options existed for their patients suffering from COPD, new data suggest that this is changing as studies are exploring better relief of symptoms, reduction of exacerbations, and reductions in mortality. The recent American Thoracic Society (ATS) Guidelines echo this in their definition of COPD as “a preventable and treatable [emphasis added] disease state characterized by airflow limitation that is not fully reversible.”[3] This increase in optimism, paired with the availability of more effective medications than were previously available to treat the disease, has given healthcare providers hope that they can now provide better outcomes to patients with COPD associated with chronic bronchitis.
COPD is a complex disease with numerous components (including bronchoconstriction, inflammation, structural changes, mucocilliary dysfunction, and systemic effects) contributing to the clinical manifestations of the disease. For this reason, pharmacologic therapy of COPD may include bronchodilators, inhaled corticosteroids (ICS), or, frequently, a combination of these classes of medications. The increasing recognition of inflammation as a key component of the disease is supported by the ATS, which now states that “the airflow limitation (in COPD) is usually progressive and is associated with an abnormal inflammatory response of the lungs.”[3]
The US Food and Drug Administration's approval of FSC 250/50 for use in COPD associated with chronic bronchitis has given healthcare providers an important therapeutic option for their patients. Of note, FSC 250/50 has been proven superior to the long-acting bronchodilator salmeterol,[4] demonstrating the substantial contribution of the ICS component of the product to improving lung function. This concept is supported in treatment guidelines for COPD, which state that utilizing 2 medications from different therapeutic classes is a convenient method to provide greater improvements in pulmonary function.[3]
Patient experience trials can provide valuable insights into individual perceptions of disease management by collecting real-world data at the patient level. Gleaning patient-reported data on how they interpret the efficacy of the regimen allows healthcare providers to understand what impact the medication has on outcomes that are especially meaningful to patients themselves (changes in breathing, compliance, and satisfaction). Positive patient perceptions of these outcomes will encourage the patient to continue taking the medication and will allow the healthcare provider to support the patient in that effort.
This initial experience trial in patients with COPD was designed to provide an opportunity whereby physicians could receive useful feedback from their patients regarding their experience with FSC 250/50 and patients could become more engaged in the management of their disease with FSC 250/50. The trial collected patient perception data on newly initiated therapy with FSC and relayed these data back to the prescribing physician.
Methods
Data for this study were obtained from patient surveys that included questions related to patients' experiences with FSC 250/50 via the DISKUS (GlaxoSmithKline, Research Triangle Park, North Carolina) device. The surveys were conducted from September 2004 through April 2005 with patients throughout the United States.
A total of 5936 primary care and specialty physicians participated in the study. Participating providers identified patients with COPD from their practices who were candidates for treatment with FSC 250/50. Eligible patients had a diagnosis of COPD associated with chronic bronchitis as determined by their physician. Patients were either newly diagnosed with COPD associated with chronic bronchitis or were still experiencing breathing difficulties on an anticholinergic medication. Selected patients were offered materials that described the study and provided instructions for how to enroll and participate in the study. Patients received a 1-month supply of FSC 250/50, delivered via the DISKUS device, and an albuterol inhaler for rescue use. After providing consent, patients who wished to participate were asked to complete 3 surveys: at baseline (before using study medication), at 2 weeks post medication initiation, and at 30 days post medication initiation. Surveys could be completed via the telephone using interactive voice response technology or via the Internet using a secure Web address. Clinical personnel on the study team designed the survey questions to elicit responses to clinically relevant health outcomes. Using touch-tone telephones, patients responded to survey questions by pressing the appropriate buttons on the telephone keypad. To complete the follow-up surveys, patients were asked to enter selected information to verify their record and permit them to continue with the surveys. All responses were recorded in a secure electronic database.
Survey 1 included questions about the duration of the condition and behaviors such as smoking. Participants were also asked to rate, on a scale of 1 (very dissatisfied) to 9 (very satisfied), their satisfaction with prior treatment. In Surveys 2 and 3, patients were asked about prior and concurrent medication use, compliance with FSC 250/50 treatment, changes in breathing overall and in the morning, convenience of FSC 250/50, satisfaction with FSC 250/50, and intent to continue its use. This paper reports data provided by patients who responded to all 3 surveys.
Survey responses from all completed surveys were summarized and reported as frequency distributions or average ratings as appropriate. A paired t-test was employed to assess the statistical significance of differences in average ratings for satisfaction with prior medication vs with FSC 250/50. Analyses were conducted using SPSS v. 11.5.0 statistical software.
Results
Patients
Approximately 1000 primary care physicians enrolled at least 1 patient in the trial. The total number of patients enrolled was 1567, and 1564 of these patients responded to Survey 1. Survey 2 was completed by 590 of the 1567 patients enrolled in the trial. Nine hundred seventy-three patients responded to Survey 3. Five hundred sixteen patients completed all 3 surveys. The mean time between Survey 1 and Survey 2 was 12 days. The mean time between Survey 1 and Survey 3 was 51 days. Baseline characteristics collected at Survey 1 are reported in Table 2.
Table 2.
Baseline Demographics
| Patients Enrolled (n=516) | |
|---|---|
| Mean age, years | 61 |
| Gender, % female | 63 |
| Length of COPD diagnosis, % of population | |
| < 1 year | 36 |
| 1-3 years | 26 |
| > 3 years | 37 |
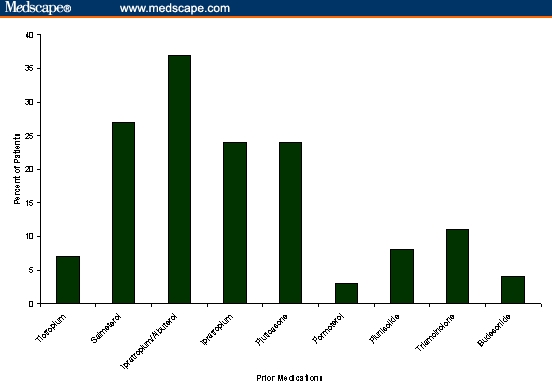
Fifty-eight percent (n=301) reported using at least 1 prescription medication for their breathing every day. The most commonly reported prior medications included ipratropium/albuterol (36%), salmeterol (26%), fluticasone (23%), and ipratropium (24%) (Figure 1).
Figure 1.
Medications prior to initiating FSC 250/50.*
* Patients may have reported more than 1 prior medication
Patient-Rated Satisfaction, Compliance, and Convenience
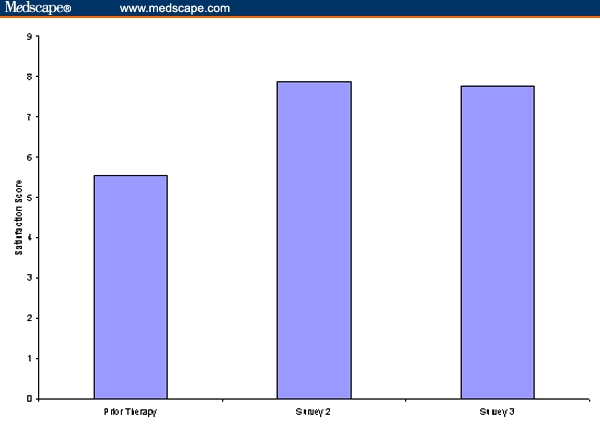
Patient satisfaction with prior therapy was rated on a 1 (very dissatisfied) to 9 (very satisfied) point scale. On average, patients reported a moderate level of satisfaction with their prior therapy, but 21% of patients said they were quite dissatisfied, answering between 1 and 3 on the 9-point scale. Results of Survey 2 and Survey 3 showed that patients experienced a high level of satisfaction with their therapy at 12 and 51 days since initiating FSC 250/50, respectively. Satisfaction scores are shown in Figure 2.
Figure 2.
Patient-rated satisfaction scores.
Furthermore, less than 4% of patients responded that they were “quite dissatisfied” with their FSC 250/50 therapy at either Survey 2 or Survey 3 (< 3 on a 9-point scale).
Patients reported a high level of compliance both at Survey 2 and Survey 3. Following an average of 12 days of therapy, 90% answered that they were taking FSC 250/50 as prescribed by their physician. This level of patient-reported compliance was unchanged after an average of 51 days on therapy, with 90% responding that they were taking their medication as prescribed by the physician. At Survey 3, 87% of the patients indicated that they intended to continue taking FSC 250/50 after the trial ended.
Convenience of FSC 250/50 was also assessed on a 1 (not at all convenient) to 9 (very convenient) point scale in the surveys. Patients were asked how convenient the FSC 250/50 inhaler was to use. The mean convenience rating for FSC 250/50 was 8.25 on a 9-point scale. Less than 3% of patients rated convenience at 3 or below, while 70% rated convenience at 9, the highest rating.
Patient-Rated Impact of FSC 250/50 on Breathing
At Survey 2, an average of 12 days after therapy with FSC 250/50 was initiated, patients reported that their breathing was very much improved since beginning the therapy, with a mean score of 6.70 on the 9-point scale. These early benefits in improved breathing were maintained after an average of 51 days on FSC 250/50. On a 1 (very much worse) to 9 (very much improved) scale, the mean response at 51 days was 7.06 with respect to the change in breathing overall. The percentage of patients reporting that their breathing was very much improved (7–9 on the 9-point scale) was considerable at Survey 2 and was maintained at Survey 3 (Figure 3). Less than 4% reported that their breathing was “very much worse” since initiation FSC 250/50 therapy at either Survey 2 or Survey 3.
Figure 3.
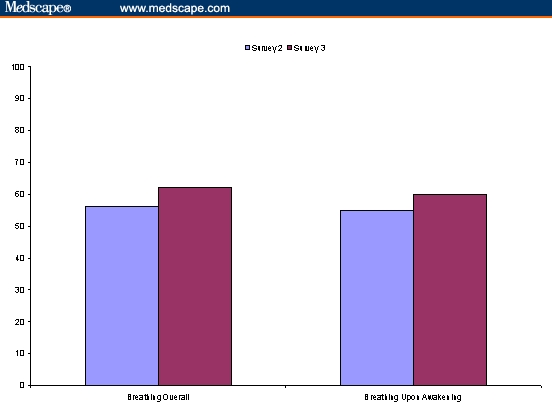
Percent of patients reporting breathing overall was very much improved after initiating FSC 250/50.
When specifically asked about the change in breathing when patients awoke in the morning, the mean response at 12 days was 6.56 on a 9-point scale, which was sustained throughout the trial with a mean score of 6.98 after 51 days. The percentage of patients reporting that their breathing upon awakening was “very much improved” followed a similar pattern to that reported for changes in breathing overall (Figure 3). Fewer than 7% of patients reported that their breathing upon awakening was “very much worse” (1–3 on the 9-point scale).
Smoking Behaviors
Information on patient smoking behaviors was collected at Survey 1. Seventy-five percent of the patients enrolling in the trial reported having smoked in the past, but 67% of this group reported that they no longer smoked. Among those who indicated they were still smoking an average of 51 days post treatment initiation with FSC 250/50, 73% reported that they would consider a quit attempt with an over-the-counter smoking cessation aid.
Discussion
Patient experience trials can provide valuable insights into individual perceptions of disease management by collecting real-world data at the patient level. Providing feedback to healthcare professionals on outcomes that patients perceive as meaningful and relevant can help the practitioner get the patient involved in their care and buy into a new treatment regimen. In this trial, patients with COPD newly prescribed FSC 250/50 were surveyed regarding their perceptions of the medication regimens before and after FSC 250/50 was initiated. Results showed positive patient perceptions in several areas, including satisfaction, compliance, convenience, and change in breathing. The positive patient experiences and improved outcomes may result in the patient becoming more comfortable with the treatment regimen, and in turn more compliant with the regimen. These results also give healthcare providers who are initiating patients on therapy with FSC 250/50 a real-world patient-level appraisal of its effectiveness so that they can set expectations of future patients starting the therapy.
Patient satisfaction with newly initiated FSC 250/50 therapy was rated high at Survey 2, less than 2 weeks after therapy began, and was sustained throughout the duration of the trial. Increased patient satisfaction with therapy has been correlated with improved compliance and better clinical outcomes.
In a 6-month study by Hanania and colleagues,[4] FSC 250/50 was shown to provide significant improvements in lung function, as measured by both predose and 2-hour postdose forced expiratory volume in 1 second (FEV1). While spirometric measurements are helpful in determining the efficacy of a management plan in COPD, a more important consideration to an individual patient is his/her own subjective assessment of the management of the disease. This study did not collect spirometric data but did assess the individual patient's perceptions of changes in breathing. Clearly, from the results of this trial, patients perceived a positive change in breathing within 2 weeks of initiating therapy with FSC 250/50. Furthermore, the positive changes in breathing were sustained throughout the trial. These findings are consistent with published data that show an increase in pulmonary function (2-hour postdose forced expiratory volume in 1 second) as early as Day 1 of FSC 250/50 treatment, which was sustained over a 6-month treatment period.[4]
COPD is a disease of multiple components, including bronchoconstriction, inflammation, and structural changes. The treatment guidelines for COPD established by the ATS indicate that combining medications from different classes is a convenient way of delivering treatment and obtaining better results, such as better lung function.[3] FSC 250/50 contains both an ICS and a long-acting bronchodilator, and has been proven superior to salmeterol,[4] another very effective medication for the management of COPD. Patients in this trial experienced perceptible improvements in several parameters that are meaningful at the individual level, including a change in breathing since beginning therapy, showing the clinical benefit of initiating therapy with FSC 250/50.
Research has shown that simplicity of a medication regimen correlates directly with adherence.[5] Patients in this experience trial rated the FSC 250/50 inhaler very convenient to use, with a mean score of 8.25 out of 9 points. A previous study conducted in Europe evaluated the ease of use of the DISKUS device.[6] In this study, 58% to 69% of patients did not experience any problems when using the DISKUS device for the first time. Patients also found the DISKUS to rank very high on the 3 most important attributes for an inhaler device: quick to use, overall ease of use, and knowing how many doses are left. Because FSC 250/50 is perceived as very convenient at the patient level, it may encourage compliance.
There are some inherent limitations in the design of this patient experience trial. A key limitation may be the lower number of responses to the optional surveys after the baseline survey. Fifteen hundred sixty-four patients responded to Survey 1, but only 590 responded to Survey 2 and 973 responded to Survey 3. This limited the number of patients responding to all 3 surveys to 516 and may have led to a selection bias for patients reporting more positive results. A second limitation is the lack of an objective measure of drug effectiveness to confirm subjective patient-rated outcomes. Even still, the results from this real-world patient experience trial provide important information that should be considered along with data from controlled clinical trials evaluating the efficacy of FSC 250/50 in patients with COPD.
In summary, patient perceptions of their medication regimen are very important. Patients reported a high level of satisfaction, compliance, and convenience with FSC 250/50 within 2 weeks of initiating therapy that was maintained over the trial period. This was accompanied by positive changes in breathing, including improvements in the ability to breathe upon awakening in the morning. These perceptions may lead to increased compliance, and therefore improved clinical outcomes. When making decisions regarding pharmacologic treatments, physicians should take into account factors such as patient-perceived ease of use, compliance, and efficacy.
Given the results seen in this patient experience trial, in patients newly diagnosed with COPD associated with chronic bronchitis, or those who are still experiencing breathing difficulties on an anticholinergic medication, FSC 250/50 provides an important therapeutic option.
Table 1.
Patient-Rated Outcomes
| Patient-Rated Outcome | Survey 2 (n=516) | Survey 3 (n=516) |
|---|---|---|
| Satisfaction with FSC 250/50* | 7.93 | 7.79 |
| Compliance with FSC 250/50 | 91% | 90% |
| Breathing “much improved”† | 57% | 70% |
| Breathing on awakening “much improved” | 55% | 67% |
Mean rating on 1 to 9 point scale (9 = very satisfied)
Rating of 7, 8, or 9 (9 = very much improved)
Contributor Information
Stuart Stoloff, The University of Nevada School of Medicine, Reno, Nevada.
Steven Samuels, Community Hospital South, Indiana Internal Medicine, Indianapolis, Indiana.
Donna Kerney, InfoMedics, Woburn, Massachusetts.
Christy P. Brown, GlaxoSmithKline, Research Triangle Park, North Carolina.
References
- 1.World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. doi: 10.1080/1357628031000116808. Available at: http://www.who.int/whr/2002/en/index.html. Accessed February 27, 2005. [DOI] [PubMed]
- 2.Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Morb Mortal Wkly Rep. 2002;51(SS-6):1–16. [PubMed] [Google Scholar]
- 3.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 4.Hanania NA, Darken P, Horstman D, et al. The efficacy and safety of fluticasone propionate 250 mcg/salmeterol 50 mcg combined in the Diskus inhaler for the treatment of chronic obstructive pulmonary disease. Chest. 2003;124:834–843. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg RN. Overview of patient compliance with medication dosing: a literature review. Clin Ther. 1984;6:592–599. [PubMed] [Google Scholar]
- 6.Moore A, Stone S. Meeting the needs of patients with COPD: patients' preference for the Diskus inhaler compared with the Handihaler. Int J Clin Pract. 2004;58:444–450. doi: 10.1111/j.1368-5031.2004.00123.x. [DOI] [PubMed] [Google Scholar]


