Abstract
Chromosomal abnormalities occur in 0.1% to 0.2% of live births, and the most common clinically significant aneuploidy among live-born infants is Down syndrome (trisomy 21). Other sonographically detectable aneuploidies include trisomy 13, 18, monosomy X, and triploidy. Second-trimester ultrasound scan detects 2 types of sonographic markers suggestive of aneuploidy. Markers for major fetal structural abnormalities comprise the first type; the second type of markers are known as “soft markers” of aneuploidy. These latter markers are nonspecific, often transient, and can be readily detected during the second-trimester ultrasound. The most commonly studied soft markers of aneuploidy include a thickened nuchal fold, rhizomelic limb shortening, mild fetal pyelectasis, echogenic bowel, and echogenic intracardiac focus and choroid plexus cyst. There is a great deal of interest in the ultrasound detection of aneuploidy, as evidenced by the large number of publications in the literature on this topic.
Unfortunately, studies evaluating the significance of the soft markers of aneuploidy vary widely and show contradictory results. In this article, we review the most common ultrasonographic soft markers used to screen aneuploidy and discuss ultrasonographic technique and measurement criteria for the detection of soft markers. We also review the clinical relevance of soft markers to aneuploidy risk assessment and evidence-based strategies for the management of affected pregnancies with each of these markers in light of current literature.
Introduction
Chromosomal abnormalities occur in 0.1% to 0.2% of live births.[1,2] Trisomy 21 (Down syndrome) is the most common karyotypic abnormality in live-born infants (1 per 800 live births)[3] and is a leading cause of mental retardation. Sonographic findings in fetuses with Down syndrome include both structural abnormalities and nonstructural abnormalities or “markers.”[4–6] Other sonographically detectable aneuploidies include trisomy 13, trisomy 18, monosomy X, and triploidy.
Various methods have been used to identify women at risk of carrying a fetus with trisomy 21, including consideration of maternal age,[2] biochemical markers,[7] amniocentesis,[8,9] and prenatal ultrasound. Amniocentesis can reliably determine fetal karyotype, but there is a 0.5% to 1.0% fetal mortality rate associated with this procedure.[8,9]
A second-trimester ultrasound scan is usually done at 18 to 22 weeks. Two types of sonographic markers suggestive of aneuploidy can be observed in the second trimester. Major fetal structural abnormalities comprise the first type (Table). There are many other, less-defined features that have been given less significance as “possible markers” of aneuploidy, and these are collectively called “soft markers” of aneuploidy (Table). Although not pathologic themselves, these markers have been used to screen for, or adjust the risk for, Down syndrome and other aneuploidies.[10,11] Soft markers may be seen in the normal fetus but have an increased incidence in infants with chromosomal abnormalities. These markers are nonspecific, often transient, and can be readily detected during the second-trimester ultrasound.[12] Thus, prenatal ultrasonography during the second trimester provides a “genetic sonogram” that is used to identify morphologic features of fetal Down syndrome.[13]
Table 1.
Major and Soft Markers of Aneuploidy
| Organ System | Major | Minor/Soft Markers |
|---|---|---|
| CNS | Ventriculomegaly | Choroid plexus cyst |
| Holoprosencephaly | ||
| Microcephaly (biparietal diameter (BPD) < 1st percentile and HP/FL < 2.5th percentile) | ||
| Dysgenesis of corpus callosum | ||
| Abnormal posterior fossa- dandy walker complex | ||
| Musculoskeletal | Hand and feet anomalies– syndactyly, clinodactyly, clenched fist, radial ray aplasia, clubfoot and rocker-bottom foot | Short long bones |
| Face | Cleft palate and lips, micrognathia, macroglossia, hypo- and hypertelorism, low set ears, small ear | – |
| Neck | Cystic hygroma | Nuchal fold thickening |
| Cardiac | Endocardial cushion defect, ventricular septal defect, hypoplastic left heart syndrome, tetralogy of Fallot, and other complex cardiac anomalies | Echogenic focus within heart |
| Gastrointestinal tract | Esophageal and duodenal atresia, small bowel obstruction, diaphragmatic hernia and omphalocele | Echogenic bowel |
| Genitourinary tract | Moderate to severe hydronephrosis, dysplastic renal disease, and renal agenesis | Mild pyelectasis |
| Others | Intrauterine growth retardation in second trimester, hydrops | Two-vessel cord, single umbilical artery |
For a number of years, members of the ultrasound community involved in obstetric sonography have been grappling with a controversial issue centered on soft markers of aneuploidy. Major abnormalities are observed in fewer than 25% of affected fetuses in most studies,[4,14–16] whereas 1 or more soft markers may be observed in at least 50% of cases.[14,17,18] Prenatal ultrasound attempts to detect the soft markers; ultrasound in the second trimester currently diagnoses 50% to 70% of cases of Down syndrome, 70% to 100% trisomy 18,[19,20] and 90% to 100% trisomy 13.[1].
The most commonly studied soft markers of aneuploidy include a thickened nuchal fold, rhizomelic limb shortening, mild fetal pyelectasis, echogenic bowel, and echogenic intracardiac focus (EIF) and choroid plexus cyst (CPC). There is a great deal of interest in the ultrasound detection of aneuploidy, as evidenced by the large number of publications in the literature on this topic. Unfortunately, studies evaluating the significance of the soft markers of aneuploidy vary widely and show contradictory results. We review the most common ultrasonographic soft markers used to screen aneuploidy and discuss ultrasonographic technique and measurement criteria for the detection of soft markers. We also review the clinical relevance of soft markers to aneuploidy risk assessment and evidence-based strategies for the management of affected pregnancies with each of these markers in light of current literature.
Some of the sonographic markers of aneuploidy are described in the Table.
Nuchal Fold Thickening
Nuchal edema in the second trimester between 15 and 23 weeks is known as the nuchal fold. Nuchal thickening was the first of the nonstructural markers identified and remains the single most predictive sonographic marker.[12] The measurement is made in the transverse plane of the fetal head slightly off the biparietal diameter, which includes the cerebellum, occipital bone, and cavum septum pellucidum (Figure 1). The nuchal fold is measured with placement of calipers from the outer edge of occipital bone to the outer edge of the skin.[21,22] Initial studies suggested a cutoff of 6 mm,[10,23–25] although subsequent studies with ROC curve analysis suggested that 5 mm is a better single cutoff before 20 weeks.[26,27] Even more recent studies suggest that gestational age-specific criteria should be used, because nuchal thickness normally increases with gestational age.[28–30] Multiples of the median and associated likelihood ratios (LRs)1 can then be calculated for the entire range of nuchal thickness measurements.[28,29]
Figure 1.
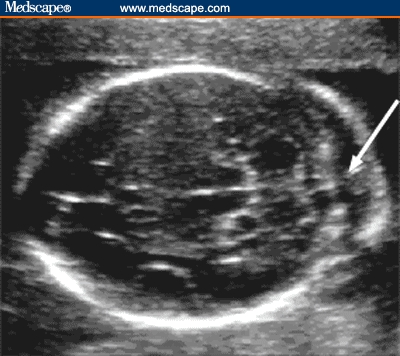
Axial image of the fetal head shows thickening of nuchal fold. Nuchal fold is measured during the second trimester on an axial image slightly off the biparietal diameter plane. The cerebellum, cisterna magna, and occipital bone should be seen. Soft tissue is measured from the outer echogenic line of occipital bone to the outer echogenic skin line.
Source: Pilu G, Nicolaides K, Ximenes R, Jeanty P. Diagnosis of fetal abnormalities. The 18-23 week scan. Diploma in Fetal Medicine. ISUOG Educational Committee. Copyright 2002 © by the authors and ISUOG. Reprinted with permission.
Echogenic Bowel
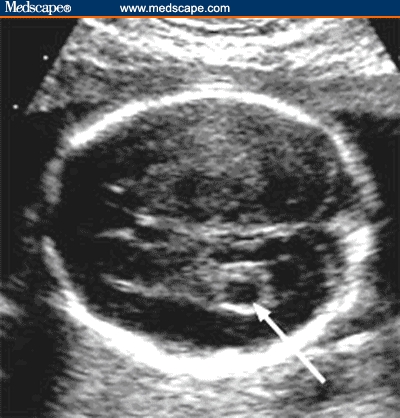
Fetal echogenic bowel refers to the presence of hyperechoic bowel, as compared with the echogenicity of the adjacent iliac bone.[31] The diagnosis of echogenic bowel is made when the bowel appears to be at least as echogenic as adjacent bone at the time of second-trimester ultrasound (Figure 2).
Figure 2.
Sagittal image of fetal abdomen shows echogenic bowel. The image should include fetal bowel, liver and iliac bone for comparison. Bowel is considered echogenic if the echogenicity of the bowel is more or equal to that of adjacent iliac bone.
Source: Pilu G, Nicolaides K, Ximenes R, Jeanty P. Diagnosis of fetal abnormalities. The 18-23 week scan. Diploma in Fetal Medicine. ISUOG Educational Committee. Copyright 2002 © by the authors and ISUOG. Reprinted with permission.
Echogenic bowel in the third trimester is a relatively common finding with uncertain clinical significance.[31] Technical factors are very important, and the frequency of the transducer should be 5 MHz or lower. Once an echogenic bowel is suspected, the gain of the ultrasound unit is lowered gradually until only bone or bowel is visible. Echogenic bowel can be classified as focal, multifocal, or diffuse. A grading system has been proposed by several authors to quantify the degree of echogenicity of fetal bowel to decrease the interobserver variation.[32,33]
Slotnick and colleagues[33] categorized echogenicity of the bowel into 3 grades, and the echogenicity of the bowel is compared with the echogenicity of the iliac crest. Grade 1 echogenic bowel refers to echogenicity of the bowel that is less than that of the iliac crest; grade 2 echogenic bowel is equal to that of the iliac crest; and grade 3 echogenic bowel is more echogenic than the iliac crest. The association of echogenic bowel with aneuploidy and adverse pregnancy outcome is strongest with moderate to severe echogenicity (grades 2 and 3).[33]
Echogenic bowel is diagnosed in 0.2% to 1.4% of all second-trimester ultrasounds.[34] It is associated with normal fetuses, fetuses with aneuploidy, intrauterine growth retardation (IUGR), bleeding, cystic fibrosis (CF), congenital viral infections, and thalassemia.[31,34–38] The association of echogenic bowel with aneuploidy, particularly trisomy 21, has been demonstrated in several studies.[34–37] The presence of echogenic bowel at the time of second-trimester ultrasound is an important finding. A detailed ultrasound of the fetus should be performed, and an amniocentesis for karyotype for evidence of cytomegalovirus (CMV), toxoplasmosis, and parvovirus infection should be recommended. CF carrier testing for both parents and maternal serologic testing of recent CMV and toxoplasmosis should also be performed (IgG and IgM).[31] Follow-up with serial growth scans is recommended, as these fetuses are at risk for IUGR.[31]
Short Long Bones
Individuals with Down syndrome can have abnormally short long bones. Fetal biometry has been used as a marker for aneuploidy, and it is recognized that the femur and humerus of fetuses with Down syndrome have a tendency to be slightly shorter compared with normal controls. Benacerraf and colleagues[39] were among the earliest investigators to describe this difference in a series of 424 patients subjected to amniocentesis.
The most common method for determination of a shortened humerus or femur is comparing the actual measurement with the expected measurement, typically on the basis of biparietal diameter or another dating parameter rather than on gestational age. The femur is considered shortened when the measured-to-expected ratio is ≤ 0.91; the humerus is considered shortened when the measured-to-expected ratio is ≥ 0.89.[40] Another approach proposed by Bahado-Singh and colleagues[41] is to calculate multiples of the median and associated LRs for the whole range of humerus length measurements, similar to the approach proposed for nuchal thickness. Femoral or humeral shortening values can be obtained using regression equations published both by Benacerraf and colleagues[39,42] and by Nyberg and colleagues.[40] Reviewing the studies, 24% to 45% of fetuses with Down syndrome had short femurs, and 24% to 54% had a short humerus; in the control population, < 5% had short long bones.[19,40,43] Some studies have also found that a shortened humerus is more predictive than a shortened femur.[12,44] The presence of short long bones that involve both the humerus and the femur seems to be less important than the finding of an isolated short humerus. This may possibly reflect the relative contribution of constitutionally small individuals.[45] A shortened humerus seems to be a better predictor than a shortened femur as reflected by the LR values of 5 and 1.5, respectively.[14]
Echogenic Intracardiac Foci
EIF are described as discrete areas of echogenicity comparable to bone in the region of papillary muscle in either cardiac ventricle.[46] The foci must be visible from different angles to make sure that one does not include specular reflections of papillary muscles.[47] EIF are found in about 1.5% to 4% of pregnancies [31,47–50] (Figure 3).
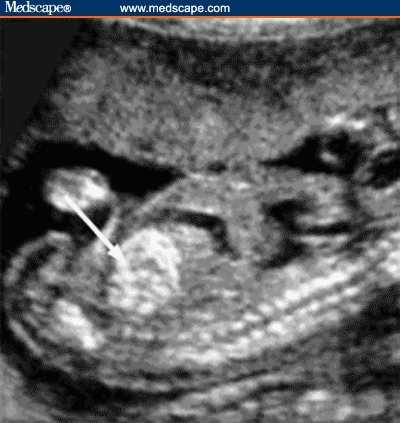
Figure 3.
Four-chamber view of the heart showing a single echogenic intracardiac focus on left side.
Source: Pilu G, Nicolaides K, Ximenes R, Jeanty P. Diagnosis of fetal abnormalities. The 18-23 week scan. Diploma in Fetal Medicine. ISUOG Educational Committee. Copyright 2002 © by the authors and ISUOG. Reprinted with permission.
Winn and colleagues[51] studied the potential misinterpretation of an echogenic focus in the heart, and they found that the rate of true EIF cases was 11 per 200 (5.5%) and the rate of false EIF cases was 34 per 200 (17%). The most common locations for identification of spurious EIF were in the moderator band, endocardial cushion, and tricuspid valve annulus.[51] These pitfalls should be kept in mind while interpreting the finding of an echogenic focus in the heart. To avoid erroneous identification and misinterpretation of EIF, recommended ultrasonographic guidelines for diagnosing a true intracardiac echogenic focus are as follows: EIF should be located within the ventricle where papillary muscles are situated; EIF should seen from more than 1 angle; EIF should be seen independent of the zone of specular reflection (Note: Be suspicious if the suspected EIF lies in this zone); and EIF should not show an entrance-exit reflection.[51]
Rochon and colleagues[31] reviewed the literature on the significance of an echogenic focus in the heart as an isolated finding and concluded that the detection of an EIF should prompt a detailed sonographic examination to search for any associated anomalies. The data that are available from low-risk populations seem to indicate, however, that an isolated focus is not associated with an increased risk of Down syndrome; or, if it is, that risk is much lower than the procedure-related loss rates associated with invasive testing. They consider an isolated echogenic focus as an incidental finding in a woman younger than 35 years of age, and amniocentesis is not recommended.[31] A similar conclusion was made in a study by Coco and colleagues,[47] who prospectively evaluated the significance of EIF in an unselected population of 12,672 women in the second trimester. The results of the statistical analysis showed that the risk of aneuploidy is increased in fetuses with an EIF.
The finding of EIF should prompt a detailed structural survey and correlation with a priori risk. Amniocentesis need not be offered to patients who are otherwise at low risk and have an isolated EIF.[47] The presence of another major or minor sign with a heart echogenic focus justifies the performance of amniocentesis.[47] In a study by Anderson and Jyoti,[50] isolated EIF in women aged 18 to 34 years was not associated with increased risk for trisomy 21 in midgestation.
A recent article addressed the issue of the EIF in a combined total of 21,839 women at low to average risk for trisomy 21.[52] Among these women, there were 626 fetuses with an isolated EIF (3%). Only 1 of the 626 with an isolated EIF had trisomy 21, a number not nearly sufficient to warrant using an isolated EIF to counsel low-risk women about chromosomal anomalies.[47,52]
Choroid Plexus Cysts
CPCs are seen in about 1% to 2.5 % of normal pregnancies as an isolated finding, and they are usually of no pathologic significance when isolated.[53–56] CPCs can be single or multiple, unilateral, or bilateral. The choroid plexus is seen in the axial plane of the head and is located in the lateral ventricle. A CPC appears as a well-circumscribed echolucent area within the choroid plexus[14] (Figure 4). The choroid plexus is homogeneous, with an echogenicity similar to soft tissue. When other anomalies are present, there is a high risk of chromosomal defects, usually trisomy 18.[54–58] The presence of CPCs does not increase the risk of trisomy 21 above the background risk.[58,59]
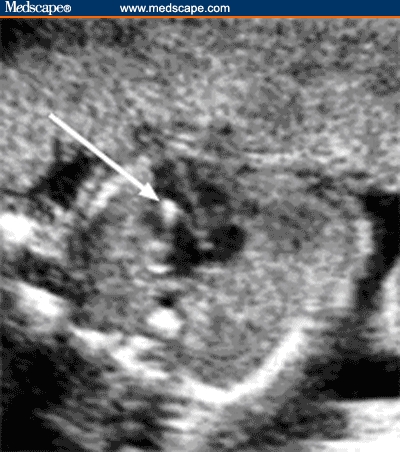
Figure 4.
Axial image of the fetal head shows a choroid plexus cyst.
Source: Pilu G, Nicolaides K, Ximenes R, Jeanty P. Diagnosis of fetal abnormalities. The 18-23 week scan. Diploma in Fetal Medicine. ISUOG Educational Committee. Copyright 2002 © by the authors and ISUOG. Reprinted with permission.
Detection of CPC warrants a detailed evaluation of fetal hands for possible overlapping digits and clenched fist to rule out trisomy 18.[54] In a large multicentric study, Chitty and colleagues[53] studied the significance of CPCs in an unselected population. There were 658 fetuses with CPCs in a total of 101,600 births. They concluded that the presence of CPCs increases the risk for aneuploidy 1.5 times, mainly trisomy 18.
Bronsteen and colleagues[54] studied 49,435 fetuses between 16 and 25 menstrual weeks; CPCs were identified in 1209 (2.3%), with 1060 cases of isolated CPC. The authors found that no fetus with an isolated CPC had trisomy 18. During the study period, 50 cases of trisomy 18 were identified between 16 and 25 menstrual weeks. CPCs were detected in half of these fetuses. They concluded that prenatal sonographic identification of CPCs warrants an extended anatomic survey that includes the fetal hands. If the fetal examination is otherwise unremarkable, then the risk for trisomy 18 is low.[54]
The probability of a chromosomal abnormality is high when CPCs are associated with any other antenatally detected anomaly, indicating a clear need to offer amniocentesis. Gupta and colleagues[60] studied a large unselected population and concluded that the predictive value of CPCs is much lower when no other anomalies are detected. They also concluded that risk did not seem to be related to whether or not cyst size diminishes as gestation progresses, whether the cysts were unilateral or bilateral, or whether they were small or large (60% to 80% < 10 mm). It is probably advisable to regard CPCs as an indication for detailed ultrasound assessment, rather than invasive testing.[60]
Mild Pyelectasis
Dilation of the fetal renal pelvis is a common finding at second-trimester ultrasound, with an incidence of 0.3% to 4.5% (average around 1%).[61–64] Mild pyelectasis is diagnosed when the renal pelvis measures ≥ 4 mm and < 10 mm in anteroposterior dimensions in axial scans of the abdomen, without caliceal dilation, in the second trimester (Figure 5).[31]
Figure 5.
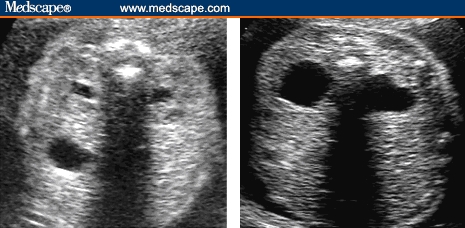
Axial images of 2 fetuses at the level of the renal pelvis show mild pyelectasis in the first image and significant pyelectasis on right side. Anterior-posterior diameter of the renal pelvis should be taken on an axial view.
Source: Pilu G, Nicolaides K, Ximenes R, Jeanty P. Diagnosis of fetal abnormalities. The 18-23 week scan. Diploma in Fetal Medicine. ISUOG Educational Committee. Copyright 2002 © by the authors and ISUOG. Reprinted with permission.
Fetuses with significant pyelectasis/hydronephrosis (≥ 10 mm) are clearly at risk for having structural abnormalities that require postnatal evaluation. Benacerraf and colleagues[63] first suggested an association of pyelectasis with aneuploidy (primarily Down syndrome) in 1990; in a selected high-risk population, 25% of fetuses with Down syndrome had mild pyelectasis compared with 2.8% of fetuses with normal karyotype.[63] The largest published series of fetal pyelectasis is an observational, prospective, multicenter study of an unselected population of 101,600 births by Chudleigh and colleagues.[65] They identified 737 fetuses with mild pyelectasis; of these 12 (1.7%) had chromosomal abnormalities. Further, 9 of these 12 fetuses had associated sonographic abnormalities, and 1 mother had advanced maternal age . Only 2 chromosomal abnormalities occurred in the setting of isolated pyelectasis in low-risk women (0.3%). The study investigators estimated the risk of aneuploidy in a fetus with isolated mild pyelectasis to be 0.33% and 2.2% in women < 36 years of age and ≥ 36 years, respectively.
A recent retrospective study reviewed the ultrasound findings of 25,586 mainly low-risk, unselected women and found 320 cases of pyelectasis with an incidence of 1.25%.[62] Nineteen of the fetuses with pyelectasis had associated sonographic anomalies; pyelectasis was an isolated finding in 301 fetuses. None of the fetuses in this series had aneuploidy. The lack of association as an isolated finding has been confirmed by other studies.[12,66,67] The results of these studies suggest that in the absence of other structural anomalies or soft markers or risk factors for aneuploidy, amniocentesis for isolated mild pyelectasis does not seem to be warranted. However, approximately one third to one quarter of fetuses show progression of their pyelectasis,[64,68] and hence the third-trimester ultrasound is recommended to identify worsening or persistent cases. The persistent pyelectasis or progression needs some degree of postnatal evaluation or surveillance.[31]
Soft Markers and Aneuploidy Risk Assessment
It has been traditional to consider an individual as being at high risk for fetal Down syndrome when the risk of aneuploidy is 1/270 or greater, which is the mid-second-trimester prevalence for a 35-year-old woman.[45] Amniocentesis is generally offered to those individuals whose risk of aneuploidy is 1/270 (at the time of amniocentesis) or greater on the basis of advanced maternal age, maternal serum screening, or both. The sonographic markers have provided a method of further evaluating the fetus for morphologic signs of trisomy 21 to further refine each patient's individual risk of having an affected fetus. Choosing which subset of the pregnant population should receive definitive karyotype determination is an important but very complex and controversial topic
We discuss this issue in the next 3 sections, specifically with regard to the importance of presence vs absence of the soft markers, isolated vs multiple markers, and marker significance in high-risk vs low-risk populations.
Importance of Absence of the Marker
The genetic sonogram, which entails a detailed search for sonographic markers of aneuploidy, can be used to identify fetuses at high risk for aneuploidy and, when normal (ie, when no sonographic markers are identified), can be used to provide evidence of a decreased risk for aneuploidy.[1] In a patient with advanced maternal age, the absence of any markers seems to be associated with a decreased risk compared with the age-related empiric risk. With a normal ultrasound, the reported associated reduction in aneuploidy risk has varied from approximately 60% to 83%.[12,69,70] In a survey of maternal-fetal medicine specialists by Egan and colleagues[71] conducted in 2002, 72% of maternal-fetal medicine physicians reported using second-trimester ultrasound to adjust aneuploidy risk; the most frequently cited risk reduction was 50%.
Importance of Presence of the Marker
Because ultrasound markers are also common among karyotypically normal fetuses, it may not be clear when genetic amniocentesis should be offered. The risk adjustment secondary to the presence of markers, and the issue of which markers are most significant, remain controversial. To help identify patients at risk, 2 ultrasound methods have been proposed.
Benacerraf and colleagues[72–74] have popularized a simple approach, referred to here as the index scoring system (ISS), whereby a score of 2 is assigned for structural defects and nuchal thickening (≥ 6 mm) and a score of 1 is assigned for the ultrasound markers EIF, echogenic bowel, pyelectasis, short femur, and short humerus. A score of 2 or more is considered positive. Using this method, the authors report a sensitivity of 73% (33 of 45 fetuses) for detecting trisomy 21, with a false-positive rate of only 4% (4 of 106 fetuses).[73] More recent modifications that also account for maternal age (score of 1 for women aged 35 to 39 years and score of 2 for women aged 40 years or older) result in a higher sensitivity (87%), but at the cost of a higher false-positive rate 27%.[74] The value of including CPCs in this system is uncertain.[74]
Using a different approach, termed the age-adjusted ultrasound risk assessment (AAURA), Nyberg and colleagues[14,75] applied LRs from ultrasound markers to the a priori risk on the basis of maternal age. This method provides patient-specific risk estimates based on maternal age, gestational age, and ultrasound findings, although it is more complicated than the ISS and requires computer calculations. By using a threshold of 1 in 200, this method has achieved a sensitivity of 74% (105 of 142) in a high-risk population.[14]
Winter and colleagues[11] designed a study to compare the accuracy of the ISS with the accuracy of the AAURA in the prenatal detection of fetal Down syndrome. In this study, 3303 consecutive women with high-risk pregnancies underwent a complete “genetic ultrasound” examination prospectively; each also had genetic amniocentesis. By using a threshold of at least 2 points to detect trisomy 21, the best ISS had a sensitivity of 45.3%, false-positive rate of 4.9%, and LR of 9.3; the positive predictive value in the high-risk population was 13.3%. Lowering the threshold to 1 point increased the sensitivity to 60.4% and increased the false-positive rate to 15.8%. Adding points for age increased the sensitivity to 67.9% but also increased the false-positive rate to 24.3%. Nearly identical results were achieved with AAURA to detect trisomy 21. At a 1 in 36 risk threshold, the sensitivity was 43.4% and the false-positive rate was 4.9%; at a 1 in 200 threshold, the sensitivity was 69.8% and the false-positive rate was 26.1%. Trisomies 18 and 13 were detected with sensitivities of 80.0% and 100.0%, respectively, with either method. The authors concluded that the modified ISS and AAURA are equivalent in screening for Down syndrome and detect approximately half of all trisomy 21 fetuses at a 5% false-positive rate.
Isolated vs Multiple Markers
Sonographic markers are considered isolated when they are not associated with major abnormalities or any other of the markers evaluated. Nyberg and colleagues[12] compared second-trimester (14 to 20 weeks) sonographic findings in 186 trisomy 21 fetuses with a control group of 8728 consecutive control fetuses through the evaluation of nuchal thickening, hyperechoic bowel, shortened femur, shortened humerus, EIF, and renal pyelectasis.
The authors reported that an isolated soft marker was the only sonographic finding in 42 (22.6%) of 186 fetuses with trisomy 21 compared with 987 (11.3%) of 8728 control fetuses (P < .001). Nuchal thickening (P < .001; LR, 11) and hyperechoic bowel (P < .001; LR, 6.7) showed the strongest association with trisomy 21 as isolated markers, followed by shortened humerus (LR, 5.1), EIF (LR, 1.8), shortened femur (LR, 1.5), and pyelectasis (LR, 1.5). EIF was the single most common isolated marker in both affected fetuses (7.1%) and control fetuses (3.9%), but carried a low risk (P = .046; LR, 1.8).[12]
Bromley and colleagues[22] studied 164 fetuses with Down syndrome detected by karyotype. They evaluated the significance of the sonographic markers as both isolated and nonisolated findings and calculated the LRs. The most sensitive sonographic markers for trisomy 21 included the nuchal fold, short femur, and an EIF. However, the false-positive rate was also the highest for a short femur and an EIF, resulting in lower LRs. Of all the sonographic markers, any finding of a nuchal fold carried the highest LR for trisomy 21. A short humerus carried the second highest LR for Down syndrome. A short humerus was identified in 48.7% of fetuses with Down syndrome compared with 2.1% of control fetuses, yielding an LR of 23.5. Major structural anomalies were found in 44 (26.8%) of 164 fetuses with Down syndrome compared with 8 (1.2%) of 656 control fetuses, yielding an LR of 22. As isolated findings, the femoral length, pyelectasis, and EIF have low LRs because of the similar prevalence of the isolated markers in the euploid population compared with the population with trisomy 21.
As an isolated finding, the nuchal fold retained the highest LR for aneuploidy; however, it was isolated only 8% of the time when it was present. An isolated short humerus had the next highest LR for aneuploidy (5.8) and was isolated just 6% of the time when it was seen. As isolated findings, the femoral length, pyelectasis, and EIF have low LRs because of the similar prevalence of the isolated markers in the euploid population compared with the population with Down syndrome. These findings suggest that the markers with the highest LRs for Down syndrome more often are clustered with other markers and are present in isolation in only a few instances. The authors concluded that the presence of nuchal fold, a structural anomaly, and a short humerus were considered sufficient to exceed the commonly accepted threshold for offering amniocentesis.
Bromley and colleagues[22] suggest that the presence of several markers that might not be of concern in isolation carries much more importance when they occur in aggregates. The presence of 2 or more of these markers resulted in an LR of 14. They concluded that clusters of markers seem to confer a higher risk of aneuploidy.[22] Similar conclusions were made by Sohl and colleagues[76] in a study of 104 fetuses with abnormal karyotype; they concluded that the presence of multiple markers (≥ 2) increases the risk for aneuploidy 12-fold.
High-Risk vs Low-Risk Population
The soft markers for Down syndrome were originally described to help improve the sonographic detection of Down syndrome in high-risk women (predominantly pregnant women of advanced maternal age) who wanted more accurate risk information than that based on age alone before deciding whether or not to undergo amniocentesis.[76] A normal ultrasound scan has been used as evidence for a reduced risk of Down syndrome in those women older than 35 years who wish to avoid amniocentesis.[14,18,66,77] For example, Nyberg and colleagues[14] concluded that a normal ultrasound scan is associated with an approximately 60% reduced risk of Down syndrome, and Nadel and colleagues[18] calculated that the probability of having a fetus with autosomal trisomy decreases from 18.8 in 1000 pregnancies to 5.3 in 1000 pregnancies for a 40-year-old woman with a normal ultrasound scan.
Available data suggest that sonographic findings are independent of maternal age and biochemical markers,[78,79] and, therefore, sonographic assessment might be applicable to low-risk patients. However, caution should be exercised in applying LRs to low-risk populations.[12] The importance and optimal course of action in a low-risk patient with a marker on prenatal sonography are controversial and not well established. If an isolated marker with an LR close to 1 is found (eg, a short femur, EIF, or pyelectasis), the patient's risk of having an affected fetus changes only minimally from her a priori risk and is probably not clinically relevant.[22] If a patient at low risk is found to have a thickened nuchal fold, a major anomaly, a short humerus, or an aggregate of markers, the pattern of findings may result in a high enough LR that the revised risk estimate exceeds the commonly accepted threshold for offering amniocentesis, and the procedure should be offered to the patient when prenatal diagnosis is desired.[22] Conversely, ultrasound assessment is probably most useful in low-risk women younger than 35 years as it identifies approximately half of fetuses affected with Down syndrome with an acceptable false-positive rate.[14]
The importance of detection of soft markers of aneuploidy is greater among high-risk women in whom high sensitivity and positive predictive value are desirable. On the other hand, the false-positive rate may be unacceptably high (13% to 17%) if any one of a panel of markers is detected in low-risk women.[14,18,78,80]
Conclusion and Summary
Sonography cannot be used to diagnose or exclude aneuploidy. It provides a noninvasive means by which to adjust the a priori risk on the basis of a variety of sonographic features. Although the literature is studded with studies on the soft markers of aneuploidy, most are done on high-risk populations. To extrapolate the findings to low-risk populations is neither scientific nor logical. Prospective studies should be conducted to confirm the value of isolated “soft markers” in low-risk women.
Although the management of each of the soft markers is different, a few generalizations can be made. First, the detection of any abnormal finding on ultrasound should prompt an immediate detailed ultrasound evaluation of the fetus by an experienced sonographer. If there is > 1 abnormal finding on ultrasound, if the patient is older than 35 years of age, or if the multiple marker screen is abnormal, an amniocentesis should be recommended to rule out aneuploidy.
If CPC or EIF is detected as an isolated marker on a second-trimester sonogram in a patient otherwise considered at low risk for fetal aneuploidy, amniocentesis is not indicated. In these circumstances, a CPC or an EIF should be considered a normal variant and is not considered clinically significant. Nuchal fold thickening, short humerus, or a major structural anomaly – even as an isolated finding – confers a high enough risk of aneuploidy in both high- and low-risk populations to recommend an amniocentesis. Echogenic bowel in isolation or in low-risk women needs a battery of investigations to rule out aneuploidy, CF, and viral infections.
Although there is ongoing debate regarding the clinical use of these markers in low-risk patients, their use in high-risk patients who have normal sonographic findings has been gaining momentum.
Footnotes
The likelihood ratio (LR) is defined as sensitivity/false-positive rate. An LR of > 1 suggests a positive association with a particular finding.
Contributor Information
Sameer Raniga, Department of Radiology, S.S.G. Hospital and Medical College, Baroda, India. Email: samhet10200@yahoo.com.
P.D. Desai, Department of Obstetrics and Gynecology, S.S.G. Hospital and Medical College, Baroda, India.
Hetal Parikh, Department of Obstetrics and Gynecology, S.S.G. Hospital and Medical College, Baroda, India.
References
- 1.Shipp TD, Benacerraf BR. Second trimester ultrasound screening for chromosomal abnormalities. Prenat Diagn. 2002;22:296–307. doi: 10.1002/pd.307. [DOI] [PubMed] [Google Scholar]
- 2.Adams MM, Erickson JD, Layde PM, Oakley GP. Down's syndrome: recent trends in the United States. JAMA. 1981;246:758–760. doi: 10.1001/jama.246.7.758. [DOI] [PubMed] [Google Scholar]
- 3.Thompson M, McInnes R, Willard H. Thompson and Thompson Genetics in Medicine. 5th ed. Philadelphia, Pa: WB Saunders; 1991. [Google Scholar]
- 4.Nyberg DA, Resta RG, Luthy DA, Hickok DE, Mahony BS, Hirsch JH. Prenatal sonographic findings of Down syndrome: review of 94 cases. Obstet Gynecol. 1990;76:370–377. [PubMed] [Google Scholar]
- 5.Benacerraf BR, Neuberg D, Bromley B, Frigoletto FD., Jr Sonographic scoring index for prenatal detection of chromosomal abnormalities. J Ultrasound Med. 1992;11:449–458. doi: 10.7863/jum.1992.11.9.449. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaides KH, Shawwa L, Brizot M, Snijders RJ. Ultrasonographically detectable markers of fetal chromosomal defects. Ultrasound Obstet Gynecol. 1993;3:56–59. doi: 10.1046/j.1469-0705.1993.03010056.x. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald ML, Wagner RM, Slotnick RN. Sensitivity and specificity of screening for Down syndrome with alpha-fetoprotein, hCG, unconjugated estriol, and maternal age. Obstet Gynecol. 1991;77:63–68. [PubMed] [Google Scholar]
- 8.The Canadian Early and Mid-trimester Amniocentesis Trial (CEMAT) Group. Randomized trial to assess safety and fetal outcome of early and mid-trimester amniocentesis. Lancet. 1998;351:242–247. [PubMed] [Google Scholar]
- 9.Tabor A, Philip J, Madsen M, et al. Randomized controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet. 1986;1:1287–1293. doi: 10.1016/s0140-6736(86)91218-3. [DOI] [PubMed] [Google Scholar]
- 10.Benacerraf BR, Gelman R, Frigoletto FD., Jr Sonographic identification of second-trimester fetuses with Down's syndrome. N Engl J Med. 1987;317:1371. doi: 10.1056/NEJM198711263172203. [DOI] [PubMed] [Google Scholar]
- 11.Winter TC, Uhrich SB, Souter VL, Nyberg DA. The “genetic sonogram”: comparison of the index scoring system with the age-adjusted US risk assessment. Radiology. 2000;215:775–782. doi: 10.1148/radiology.215.3.r00ma36775. [DOI] [PubMed] [Google Scholar]
- 12.Nyberg DA, Souter VL, El-Bastawissi A, Young S, Luthhardt F, Luthy DA. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. J Ultrasound Med. 2001;20:1053–1063. doi: 10.7863/jum.2001.20.10.1053. [DOI] [PubMed] [Google Scholar]
- 13.Benacerraf BR. Use of sonographic markers to determine the risk of Down syndrome in second-trimester fetuses (editorial) Radiology. 1996;201:619–620. doi: 10.1148/radiology.201.3.8939206. [DOI] [PubMed] [Google Scholar]
- 14.Nyberg DA, Luthy DA, Resta RG, Nyberg BC, Williams MA. Age-adjusted ultrasound risk assessment for fetal Down's syndrome during the second trimester: description of the method and analysis of 142 cases. Ultrasound Obstet Gynecol. 1998;12:8–14. doi: 10.1046/j.1469-0705.1998.12010008.x. [DOI] [PubMed] [Google Scholar]
- 15.Stoll C, Dott B, Alembik Y, Roth MP. Evaluation of routine prenatal ultrasound examination in detecting fetal chromosomal abnormalities in a low risk population. Hum Genet. 1993;91:37–41. doi: 10.1007/BF00230219. [DOI] [PubMed] [Google Scholar]
- 16.Hill LM. The sonographic detection of trisomies 13, 18, and 21. Clin Obstet Gynecol. 1996;39:831–850. doi: 10.1097/00003081-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Vintzileos AM, Campbell WA, Guzman ER, Smulian JC, McLean DA, Ananth CV. Second-trimester ultrasound markers for detection of trisomy 21: which markers are best? Obstet Gynecol. 1997;89:941–944. doi: 10.1016/s0029-7844(97)00152-x. [DOI] [PubMed] [Google Scholar]
- 18.Nadel AS, Bromley B, Frigoletto FD, Jr, Benacerraf BR. Can the presumed risk of autosomal trisomy be decreased in fetuses of older women following a normal sonogram? J Ultrasound Med. 1995;14:297–302. doi: 10.7863/jum.1995.14.4.297. [DOI] [PubMed] [Google Scholar]
- 19.Yeo L, Guzman ER, Day-Salvatore D, Walters C, Chavez D, Vintzileos AM. Prenatal detection of fetal trisomy 18 through abnormal sonographic features. J Ultrasound Med. 2003;22:581–590. doi: 10.7863/jum.2003.22.6.581. [DOI] [PubMed] [Google Scholar]
- 20.DeVore GR. Second trimester ultrasonography may identify 77 to 97% of fetuses with trisomy 18. J Ultrasound Med. 2000;19:565–576. doi: 10.7863/jum.2000.19.8.565. [DOI] [PubMed] [Google Scholar]
- 21.Stewart T. Screening for aneuploidy: the genetic sonogram. Obstet Gynecol Clin North Am. 2004;31:21–33. doi: 10.1016/S0889-8545(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 22.Bromley B, Lieberman E, Shipp TD, Benacerraf BR. The genetic sonogram - a method of risk assessment for Down syndrome in the second trimester. J Ultrasound Med. 2002;21:1087–1096. doi: 10.7863/jum.2002.21.10.1087. [DOI] [PubMed] [Google Scholar]
- 23.Benacerraf BR, Barrs VA, Laboda LA. A sonographic sign for the detection in the second trimester of the fetus with Down's syndrome. Am J Obstet Gynecol. 1985;151:1078–1079. doi: 10.1016/0002-9378(85)90385-0. [DOI] [PubMed] [Google Scholar]
- 24.Benacerraf BR, Frigoletto FD, Jr, Laboda L. Sonographic diagnosis of Down syndrome in the second trimester. Am J Obstet Gynecol. 1985;153:49–52. doi: 10.1016/0002-9378(85)90588-5. [DOI] [PubMed] [Google Scholar]
- 25.Benacerraf BR, Frigoletto FD., Jr Soft tissue nuchal fold in the second-trimester fetus: standards for normal measurements compared with those in Down syndrome. Am J Obstet Gynecol. 1987;157:1146–1149. doi: 10.1016/s0002-9378(87)80279-x. [DOI] [PubMed] [Google Scholar]
- 26.Gray DL, Crane JP. Optimal nuchal skin-fold thresholds based on gestational age for prenatal detection of Down syndrome. Am J Obstet Gynecol. 1994;171:1282–1286. doi: 10.1016/0002-9378(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 27.Borrell A, Costa D, Martinez JM, et al. Early midtrimester fetal nuchal thickness: effectiveness as a marker for Down syndrome. Obstet Gynecol. 1996;175:45–49. doi: 10.1016/s0002-9378(96)70249-1. [DOI] [PubMed] [Google Scholar]
- 28.Locatelli A, Piccoli MG, Vergani P, et al. Critical appraisal of the use of nuchal fold thickness measurements for the prediction of Down syndrome. Am J Obstet Gynecol. 2000;182:192–197. doi: 10.1016/s0002-9378(00)70512-6. [DOI] [PubMed] [Google Scholar]
- 29.Bahado-Singh RO, Oz UA, Kovanci E, et al. Gestational age standardized nuchal thickness values for estimating mid-trimester Down's syndrome risk. J Matern Fetal Med. 1999;8:37–43. doi: 10.1002/(SICI)1520-6661(199903/04)8:2<37::AID-MFM1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Tannirandorn Y, Manotaya S, Uerpairojkit B, Tanawattanacharoen S, Charoenvidhya D, Phaosavasdi S. Cut-off criteria for second-trimester nuchal skinfold thickness for prenatal detection of Down syndrome in a Thai population. Int J Gynaecol Obstet. 1999;65:137–141. doi: 10.1016/s0020-7292(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 31.Rochon M, Eddleman K. Controversial ultrasound findings. Obstet Gynecol Clin North Am. 2004;31:61–99. doi: 10.1016/S0889-8545(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 32.Nyberg DA, Dubinsky T, Resta RG, Manony BS, Hickok DE, Luthy DA. Echogenic fetal bowel during the second trimester: clinical importance. Radiology. 1993;188:527–531. doi: 10.1148/radiology.188.2.8327709. [DOI] [PubMed] [Google Scholar]
- 33.Slotnick RN, Abuhamad AZ. Prognostic implications of fetal echogenic bowel. Lancet. 1996;347:85–87. doi: 10.1016/s0140-6736(96)90210-x. [DOI] [PubMed] [Google Scholar]
- 34.Al-Kouatly HB, Chasen ST, Streltzoff J, Chervenak FA. The clinical significance of fetal echogenic bowel. Am J Obstet Gynecol. 2001;185:1035–1038. doi: 10.1067/mob.2001.117671. [DOI] [PubMed] [Google Scholar]
- 35.Al-Kouatly HB, Chasen ST, Karam AK, Ahner R, Chervenak FA. Factors associated with fetal demise in fetal echogenic bowel. Am J Obstet Gynecol. 2001;185:1039–1043. doi: 10.1067/mob.2001.117641. [DOI] [PubMed] [Google Scholar]
- 36.Bromley B, Doubilet P, Frigoletto FD, Jr, Krauss C, Estroff JA, Benacerraf BR. Is fetal hyperechoic bowel on second-trimester sonogram an indication for amniocentesis? Obstet Gynecol. 1994;83(5 Pt 1):647–651. [PubMed] [Google Scholar]
- 37.Berlin BM, Norton ME, Sugarman EA, Tsipis JE, Allitto BA. Cystic fibrosis and chromosome abnormalities associated with echogenic fetal bowel. Obstet Gynecol. 1999;94:135–138. doi: 10.1016/s0029-7844(99)00286-0. [DOI] [PubMed] [Google Scholar]
- 38.Lam YH, Tang MH, Lee CP, Tse HY. Echogenic bowel in fetuses with homozygous alpha-thalassemia-1 in the first and second trimesters. Ultrasound Obstet Gynecol. 1999;14:180–182. doi: 10.1046/j.1469-0705.1999.14030180.x. [DOI] [PubMed] [Google Scholar]
- 39.Benacerraf BR, Nyberg D, Frigoletto FD., Jr Humeral shortening in second-trimester fetuses with Down syndrome. Obstet Gynecol. 1991;77:223–227. doi: 10.1097/00006250-199102000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Nyberg DA, Resta RG, Luthy DA, Hickok DE, Williams MA. Humerus and femur length shortening in the detection of Down's syndrome. Am J Obstet Gynecol. 1993;168:534–539. doi: 10.1016/0002-9378(93)90487-4. [DOI] [PubMed] [Google Scholar]
- 41.Bahado-Singh RO, Oz AU, Kovanci E, et al. New Down syndrome screening algorithm: ultrasonographic biometry and multiple serum markers combined with maternal age. Am J Obstet Gynecol. 1998;179:1627–1631. doi: 10.1016/s0002-9378(98)70036-5. [DOI] [PubMed] [Google Scholar]
- 42.Benacerraf BR, Cnann A, Gelman R, Laboda LA, Frigoletto FD., Jr Can sonographers reliably identify anatomic features associated with Down syndrome in fetuses? Radiology. 1989;173:377–380. doi: 10.1148/radiology.173.2.2529580. [DOI] [PubMed] [Google Scholar]
- 43.Johnson MP, Michaelson JE, Barr M, Jr, et al. Combining humerus and femur length for improved ultrasonographic identification of pregnancies at increased risk for trisomy 21. Am J Obstet Gynecol. 1995;172(4 Pt 1):1229–1235. doi: 10.1016/0002-9378(95)91484-6. [DOI] [PubMed] [Google Scholar]
- 44.Rodis JF, Vintzileos AM, Fleming AD, et al. Comparison of humerus length with femur length in fetuses with Down syndrome. Am J Obstet Gynecol. 1991;165:1051–1056. doi: 10.1016/0002-9378(91)90468-7. [DOI] [PubMed] [Google Scholar]
- 45.Smith-Bindman R, Hosmer W, Feldstein V, Deeks J, Goldberg J. Second-trimester ultrasound to detect fetuses with Down syndrome: a meta-analysis. JAMA. 2001;285:1044–1055. doi: 10.1001/jama.285.8.1044. [DOI] [PubMed] [Google Scholar]
- 46.Stone JL, Eddleman KA, Berkowitz RL. The echogenic intracardiac focus. Contemp Ob/Gyn. 1998;43:73–78. [Google Scholar]
- 47.Coco C, Jeant P, Jeanty C. An isolated echogenic heart focus is not an indication for amniocentesis in 12,672 unselected patients. J Ultrasound Med. 2004;23:489–496. doi: 10.7863/jum.2004.23.4.489. [DOI] [PubMed] [Google Scholar]
- 48.Sotiriadis A, Makrydimas G, Ioannidis JP. Diagnostic performance of intracardiac echogenic foci for Down syndrome: a meta-analysis. Obstet Gynecol. 2003;101:1009–1016. doi: 10.1016/s0029-7844(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 49.Barsoom MJ, Feldman DM, Borgida AF, Esters D, Diana D, Egan JF. Is an isolated fetal cardiac echogenic focus an indication for fetal echocardiography? J Ultrasound Med. 2001;20:1043–1046. doi: 10.7863/jum.2001.20.10.1043. [DOI] [PubMed] [Google Scholar]
- 50.Anderson N, Jyoti R. Relationship of isolated fetal intracardiac echogenic focus to trisomy 21 at the mid-trimester sonogram in women younger than 35 years. Ultrasound Obstet Gynecol. 2003;21:354–358. doi: 10.1002/uog.89. [DOI] [PubMed] [Google Scholar]
- 51.Winn VD, Joy Sonson BA, Filly RA. Echogenic intracardiac focus potential for misdiagnosis. J Ultrasound Med. 2003;22:1207–1214. doi: 10.7863/jum.2003.22.11.1207. [DOI] [PubMed] [Google Scholar]
- 52.Filly RA, Benacerraf BR, Nyberg DA, Hobbins JC. Choroid plexus cyst and echogenic intracardiac focus in women at low risk for chromosomal anomalies. J Ultrasound Med. 2004;23:447–449. doi: 10.7863/jum.2004.23.4.447. [DOI] [PubMed] [Google Scholar]
- 53.Chitty LS, Chudleigh P, Wright E, Campbell S, Pembrey M. The significance of choroid plexus cysts in an unselected population: results of a multicenter study. Ultrasound Obstet Gynecol. 1998;12:391–397. doi: 10.1046/j.1469-0705.1998.12060391.x. [DOI] [PubMed] [Google Scholar]
- 54.Comstock H. Second-trimester sonography and trisomy 18 - the significance of isolated choroid plexus cysts after an examination that includes the fetal hands. J Ultrasound Med. 2004;23:241–245. doi: 10.7863/jum.2004.23.2.241. [DOI] [PubMed] [Google Scholar]
- 55.Ghidini A, Strobelt N, Locatelli A, Mariani E, Piccoli MG, Vergani P. Isolated fetal choroid plexus cysts: role of ultrasonography in establishment of the risk of trisomy 18. Am J Obstet Gynecol. 2000;182:972–977. doi: 10.1016/s0002-9378(00)70356-5. [DOI] [PubMed] [Google Scholar]
- 56.Shields LE, Carpenter LA, Smith KM, Nghiem HV. Ultrasonographic diagnosis of trisomy 18: is it practical in the early second trimester? J Ultrasound Med. 1998;17:327–331. doi: 10.7863/jum.1998.17.5.327. [DOI] [PubMed] [Google Scholar]
- 57.Feuchtbaum LB, Currier RJ, Lorey FW, Cunningham GC. Prenatal ultrasound findings in affected and unaffected pregnancies that are screen-positive for trisomy 18: the California experience. Prenat Diagn. 2000;20:293–299. doi: 10.1002/(sici)1097-0223(200004)20:4<293::aid-pd801>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 58.Bromley B, Lieberman E, Benacerraf BR. Choroid plexus cysts: not associated with Down syndrome. Ultrasound Obstet Gynecol. 1996;8:232–235. doi: 10.1046/j.1469-0705.1996.08040232.x. [DOI] [PubMed] [Google Scholar]
- 59.Yoder PR, Sabbagha RE, Gross SJ, Zelop CM. The second-trimester fetus with isolated choroid plexus cysts: a meta-analysis of risk of trisomies 18 and 21. Obstet Gynecol. 1999;93:869–872. doi: 10.1016/s0029-7844(98)00544-4. [DOI] [PubMed] [Google Scholar]
- 60.Gupta JK, Cave M, Lilford RJ, et al. Clinical significance of fetal choroid plexus cysts. Lancet. 1995;346:724–729. doi: 10.1016/s0140-6736(95)91502-8. [DOI] [PubMed] [Google Scholar]
- 61.Corteville JE, Kicke JM, Crane JP. Fetal pyelectasis and Down syndrome: is genetic amniocentesis warranted? Obstet Gynecol. 1992;79:770–772. [PubMed] [Google Scholar]
- 62.Havutcu AE, Nikolopoulos G, Adinkra P, Lamont RF. The association between fetal pyelectasis on second trimester ultrasound scan and aneuploidy among 25, 586 low-risk unselected women. Prenatal Diagn. 2002;22:1201–1206. doi: 10.1002/pd.490. [DOI] [PubMed] [Google Scholar]
- 63.Benacerraf BR, Mandell J, Estroff JA, Harlow BL, Frigoletto F. Fetal pyelectasis: a possible association with Down syndrome. Obstet Gynecol. 1990;76:58–60. [PubMed] [Google Scholar]
- 64.Ismaili K, Hall M, Donner C, Thomas D, Vermeylen D, Avni FE. Results of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am J Obstet Gynecol. 2003;188:242–246. doi: 10.1067/mob.2003.81. [DOI] [PubMed] [Google Scholar]
- 65.Chudleigh PM, Chitty LS, Prembery M, Campbell S. The association of aneuploidy and mild fetal pyelectasis in an unselected population: the result of multicenter population. Ultrasound Obstet Gynecol. 2001;17:197–202. doi: 10.1046/j.1469-0705.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 66.Vintzileos AM, Egan JFX. Adjusting the risk for trisomy 21 on the basis of second-trimester ultrasonography. Am J Obstet Gynecol. 1995;172:837–844. doi: 10.1016/0002-9378(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 67.Snijders RJ, Sebire NJ, Faria M, Patel F, Nicolaides KH. Fetal mild hydronephrosis and chromosomal defects: relation to maternal age and gestation. Fetal Diagn Ther. 1995;10:349–355. doi: 10.1159/000264258. [DOI] [PubMed] [Google Scholar]
- 68.Wickstrom E, Maizels M, Sabbagha RE, Tamura RK, Cohen LC, Pergament E. Isolated fetal pyelectasis: assessment of risk for postnatal uropathy and Down syndrome. Ultrasound Obstet Gynecol. 1996;8:236–240. doi: 10.1046/j.1469-0705.1996.08040236.x. [DOI] [PubMed] [Google Scholar]
- 69.Nyberg AS, Bromley B, Frigoletto FD, Benacerraf BR. Can the presumed risk of autosomal trisomy be decreased in fetuses of older women following a normal sonogram? J Ultrasound Med. 1995;14:297–302. doi: 10.7863/jum.1995.14.4.297. [DOI] [PubMed] [Google Scholar]
- 70.Vintrileos AM, Uzman ER, Smulian JC, Yeo L, William E, Knuppel RA. Down syndrome risk estimation after normal genetic sonography. Am J Obstet Gynecol. 2002;187:1226–1229. doi: 10.1067/mob.2002.126984. [DOI] [PubMed] [Google Scholar]
- 71.Egan JF, Kaminsky LM, DeRoche ME, Barsoom MJ, Bargida AF, Benn PA. Antenatal Down syndrome screening in the United States in 2001: a survey of maternal-fetal medicine specialists. Am J Obstet Gynecol. 2002;187:1230–1234. doi: 10.1067/mob.2002.126980. [DOI] [PubMed] [Google Scholar]
- 72.Benacerraf BR, Nyberg D, Bromley B, Frigoletto FD., Jr Sonographic scoring index for prenatal detection of chromosomal abnormalities. J Ultrasound Med. 1992;11:449–458. doi: 10.7863/jum.1992.11.9.449. [DOI] [PubMed] [Google Scholar]
- 73.Benacerraf BR, Nadel A, Bromley B. Identification of second-trimester fetuses with autosomal trisomy by use of a sonographic scoring index. Radiology. 1994;193:135–140. doi: 10.1148/radiology.193.1.8090881. [DOI] [PubMed] [Google Scholar]
- 74.Bromley B, Lieberman E, Benacerraf BR. The incorporation of maternal age into the sonographic scoring index for the detection at 14–20 weeks of fetuses with Down's syndrome. Ultrasound Obstet Gynecol. 1997;10:321–324. doi: 10.1046/j.1469-0705.1997.10050321.x. [DOI] [PubMed] [Google Scholar]
- 75.Nyberg DA, Luthy DA, Williams MA, Winter TC. Genetic sonogram: computerized assessment of risk for Down syndrome based on obstetric US findings during the second trimester. Radiology. 1996;201:160. [Google Scholar]
- 76.Sohl B, Scioscia A, Budorick NE, Moore TR. Utility of minor ultrasonographic markers in the prediction of abnormal fetal karyotype at a prenatal diagnostic center. Am J Obstet Gynecol. 1999;181:898–903. doi: 10.1016/s0002-9378(99)70322-4. [DOI] [PubMed] [Google Scholar]
- 77.Vintzileos AM, Campbell WA, Rodis JF, Guzman ER, Smulian JC, McLean DA. Second-trimester ultrasound (U/S) markers for the detection of trisomy-21: which markers are best? [abstract] J Ultrasound Med. 1997;16(3 Suppl):66. [Google Scholar]
- 78.Verdin SM, Economides DL. The role of ultrasonographic markers for trisomy 21 in women with positive serum biochemistry. Br J Obstet Gynaecol. 1998;105:63–67. doi: 10.1111/j.1471-0528.1998.tb09352.x. [DOI] [PubMed] [Google Scholar]
- 79.Nyberg DA, Luthy DA, Cheng EY, Sheley RC, Resta RG, Williams MA. Role of prenatal ultrasound in women with positive screen for Down syndrome based on maternal serum markers. Am J Obstet Gynecol. 1995;173:1030–1035. doi: 10.1016/0002-9378(95)91322-x. [DOI] [PubMed] [Google Scholar]
- 80.Vintzileos AM, Campbell WA, Rodis JF, Guzman ER, Smulian JC, Knuppel RA. The use of second trimester genetic sonogram in guiding clinical management of patients at increased risk for fetal trisomy 21. Obstet Gynecol. 1996;87:948–952. doi: 10.1016/0029-7844(96)00053-1. [DOI] [PubMed] [Google Scholar]


