Abstract
Previous clinical attempts to correct genetic deficiencies such as hemophilia or Gaucher disease by transplantation of allogeneic spleen were associated with aggressive graft versus host disease, mediated by mature T cells derived from the donor spleen. We show that a fetal pig spleen harvested at the embryonic day 42 stage, before the appearance of T cells, exhibited optimal growth potential upon transplantation into SCID mice, and the growing tissue expressed factor VIII. Transplantation of embryonic day 42 spleen tissue into hemophilic SCID mice led to complete alleviation of hemophilia within 2–3 months after transplant, as demonstrated by tail bleeding and by assays for factor VIII blood levels. These results provide a proof of principle to the concept that transplantation of a fetal spleen, obtained from a developmental stage before the appearance of T cells, could provide a novel treatment modality for genetic deficiencies of an enzyme or a factor that can be replaced by the growing spleen tissue.
Keywords: embryonic, factor VIII
Genetic disorders represent natural targets for gene therapy; nevertheless, considerable difficulties arise in targeting gene delivery to specific cell types in vivo, regulating the expression of recombinant genes, and controlling vector immunogenicity (1).
Hemophilia A is thought to be a particularly promising gene therapy target because the deficient protein (factor VIII) circulates systemically and can be synthesized, in theory, by various cell types. Indeed, preliminary trials have demonstrated expression of human factor VIII in animals and in patients after gene transfer (2–4). In parallel, progress in tissue transplantation over the past decade encouraged the consideration of cell or organ transplantation as a potential treatment for genetic diseases, such as hemophilia A.
The liver is considered to be the primary source of factor VIII protein. Hepatocytes and liver sinusoidal endothelial cells, but not Kupffer cells, produce factor VIII in the mouse liver (5). The role of the liver in factor VIII synthesis has been supported by liver transplantation studies in both hemophilic animals and humans, after which increasing factor VIII levels were detected (6–10). Early transplantation studies in the canine model (11, 12) led, in the early 1960s, to the first human spleen transplants in four patients with malignancies and one with agammaglobulinemia (13).
The feasibility of spleen transplantation in the treatment of hemophilia A in humans was first documented in 1969 by Hathaway et al. (14), who transplanted a spleen from a father to his son (14). The recipient displayed a marked rise in factor VIII shortly after the transplant, but the spleen graft had to be removed 4 days later because it ruptured and bled. Further animal studies in hemophilic animals have confirmed that organs such as the spleen and lung can contribute to the presence of circulating factor VIII (15, 16). Subsequently, a number of spleen transplants (living donor or cadaver) in hemophilic patients were reported. At least one resulted in sustained, normalized levels of factor VIII with stable factor VIII production for 5 months after operation (17, 18). Little data are available regarding spleen transplantation in the treatment of other genetic diseases, but there are several case reports in the literature suggesting the potential of spleen transplantation as a treatment for Gaucher disease (19, 20).
A major obstacle in spleen transplantation is associated with graft versus host disease (GVHD) mediated by donor T cells present in the spleen graft (18). In principle, this potentially lethal complication may be prevented if it were possible to use embryonic precursor spleen tissue obtained before the appearance of mature T cells in the spleen.
Considering the recent interest in fetal tissue as a source for transplantation, it is surprising that the role of embryonic spleen tissue as a source for secreted proteins has never been studied. Clearly, if early embryonic spleen tissue were to prove effective, it could be of particular value because this precursor tissue is devoid of T cells, which are known to mediate GVHD typical of adult spleen transplantation.
In the present study we examined, for the first time, the potential of pig embryonic spleen tissue as a novel tissue source for transplantation, with emphasis on relatively early gestational time points at which mature T cells are not present in the implant. Thus, we initially defined embryonic day (E) 42 as the optimal gestational time point that could provide tissue for transplantation based on growth potential and immunogenicity. Furthermore, the reduced immunogenicity associated with E42 embryonic pig spleen tissue has enabled achievement of engraftment in fully immune, competent recipients under a tolerable immune suppression protocol used in allogenic transplantation. Finally, the proof of concept, namely the ability to correct hemophilia using early embryonic spleen tissue, is demonstrated in factor VIII-KO SCID mice.
Results
Transplantation of Pig Embryonic Spleen Tissue into SCID Mice.
Optimal growth before T cell appearance in the embryonic spleen is attained at E42.
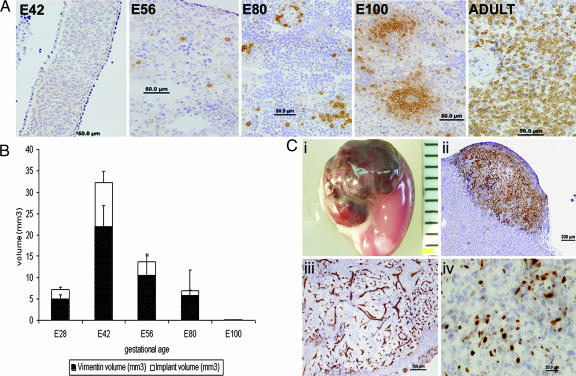
We previously established the gestational time window enabling the harvest of human and pig embryonic kidney precursor tissue for growing functional small kidneys in SCID mice (21). More recently, we defined similar “windows” for the pig embryonic pancreas, liver, and lung (22). In the present study, spleen embryonic tissue was harvested at different gestational time points and stained for CD3-positive cells to determine the precise time period in pig ontogeny at which mature T cells are first present in these tissues. As can be seen in Fig. 1A, T cells were initially detectable in the tissue obtained at the E56 gestational age. To define the earliest gestational time point at which the maximal growth potential of transplanted tissue is exhibited, pig embryonic spleen tissues obtained at different stages, ranging from E28 to E100, were implanted into NOD-SCID mice. Six weeks after implantation, the implants' sizes, as well as differentiations, were evaluated by computerized morphometric analysis. Consecutive sections were cut at 40-μm intervals and stained for vimentin using a mouse monoclonal antibody, previously shown by Bohn et al. (23) to bind vimentin of all mammalian species except for the mouse. Thus, this antibody can selectively distinguish between porcine and mouse vimentin. This selectivity is clearly illustrated in Fig. 1Cii, in which the mouse kidney area can be clearly distinguished from the porcine embryonic spleen transplant grafted under the kidney capsule. Using the Image Pro program, the total mesenchymal area in the graft was determined. As can be seen in Fig. 1B, which summarizes the results of the morphometric analysis, the largest vimentin-positive implant volume was found after transplantation of tissue obtained at E42. Tissue growth was significantly accelerated compared with implants obtained at earlier (E28) or later (E56, E80, and E100) gestational time points, suggesting unique growth potential of spleen precursors at particular time points during gestation. Thus, to avoid GVHD and maximize growth, we chose to harvest pig spleen tissue for further transplantation studies at E42.
Fig. 1.
Histological markers of pig embryonic spleen before and after implantation. (A) Immunohistological staining of pig CD3 T cells in embryonic spleen tissue harvested at E42, E56, E80, E100, and adult spleen. (B) Morphometric analysis of implant growth. Different gestational ages were evaluated for total volume (white bars) and for vimentin volume-positive tissue (black bars) 6 weeks after transplantation (n = 5). (C) Development of E42 pig spleen graft under the kidney capsule of NOD-SCID mice. (Ci) Macroscopic view of E42 graft 6 weeks after transplant. (Cii) Pig mesenchymal components stained by anti-vimentin (V9). (Ciii) Porcine blood vessels stained by anti-pig CD31. (Civ) Proliferative status of the growing implant demonstrated by ki67 staining.
A macroscopic image of the implant growing 6 weeks after transplantation of E42 pig spleen tissue is shown in Fig. 1Ci. The pig origin of the stromal component was demonstrated by vimentin immunolabeling (Fig. 1Cii). These stromal cells are supported by pig blood vessels stained by anti-CD31 (Fig. 1Ciii) (non-cross-reactive with mouse CD31) and are highly proliferative, as demonstrated by high levels of Ki67 labeling (Fig. 1Civ).
Growth and development of E42 pig spleen tissue at different time points after transplant.
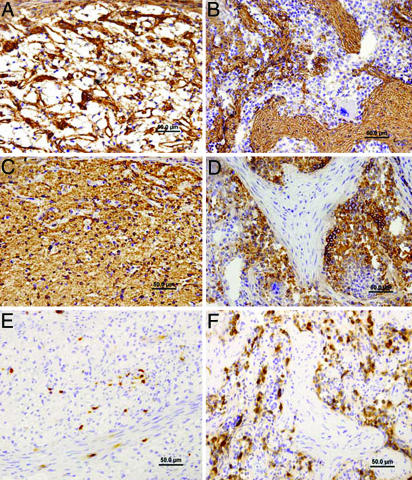
To evaluate the formation of host hematopoietic elements in the growing spleen transplant, specific antibodies directed against mouse myeloid (anti-Mac1) or erythroid (anti-TER-119) cells were used at different time points after transplant (because of the SCID mutation of the recipients, host T and B lymphocytes were not present in the transplant). This analysis revealed that hematopoietic nests, separated by connective tissue septa, appear only around the third month after transplant (Fig. 2) and fully develop at 5 months (data not shown). In these nests, hematopoietic cells were observed adjacent to loosely distributed mesenchymal stromal elements. Thus, the E42 spleen tissue gradually assumes its hematopoietic character without any signs of aberrant differentiation typical of tumors and, in particular, of teratomas.
Fig. 2.
Development of hematopoietic nests and fibrous septae in pig E42 spleen implants. At 2 months, a sponge-like fibrous reticular network outlined by anti-laminin antibody (A) with diffusely entrapped mouse erythroid cells stained by anti-mouse TER-119 (C) is seen. In contrast, at 3 months after transplant, dense laminin-positive connective tissue septa is evident (B) surrounding nests of mouse hematopoietic tissue, including TER-119-positive erythropoietic areas, regions with megakaryocytes and myelopoiesis (D). Host myeloid cells, demonstrated by mouse Mac-1 immunostaining, are diffusely distributed in spleen transplants at 2 months (E) but become numerous within hematopoietic nests at 3 months after transplant (F).
E42 pig spleens exhibit reduced immunogenicity compared with tissue harvested at E56.
While xenotransplantation generally requires relatively intensive and toxic immune suppression compared with that used in allogeneic organ transplantation, engraftment of embryonic xenografts, which have been shown to be less immunogenic, might be attained under less aggressive protocols. To evaluate whether the E42 pig spleen tissue is less immunogenic compared with tissue harvested at a later gestational age, we tested the potential of the implanted tissue to induce rejection by human lymphocytes infused i.p., in parallel to the transplantation of the spleen tissue in NOD-SCID mice. In this model, rejection of foreign tissues or cells can be induced by infusion of 80 × 106 human peripheral blood mononuclear cells (PBMC) into the peritoneum of host SCID mice (Hu-SCID), as originally described by Mosier et al. (24) and subsequently used to evaluate the immunogenicity of pig or human embryonic tissues harvested at different gestational time points (25–27).
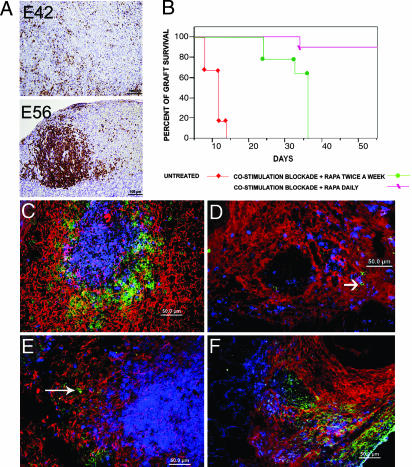
Considering that adoptively transferred human lymphocytes tend to die of apoptosis by the second month after transplant and, in accordance with previous studies that showed that optimal rejection is attained between 2 and 4 weeks after infusion of PBMC (28), we compared the growth of E42 and E56 pig spleen tissue in the presence of human PBMC at 4 weeks after transplant. As can be seen in Fig. 3A, while implants of E56 embryonic spleen tissue in the presence of human PBMC were completely rejected by 4 weeks after transplant, and massive focal infiltration by human CD45 cells was clearly documented, E42 spleen tissue was not rejected, exhibiting only minor diffused infiltration of CD45 cells in some of the grafts. Thus, the E42 embryonic spleen tissue is more resistant to rejection compared with E56 tissue.
Fig. 3.
Engraftment of pig E42 embryonic spleen in Hu-SCID and C57BL/6 immunocompetent mice. (A) Rejection pattern of pig embryonic spleen mediated by Hu-PBMCs, 4 weeks after transplantation. Anti-human CD45 staining demonstrates reduced infiltration into grafts originating from E42 compared with E56 pig spleen. (B) Engraftment of E42 spleen under different immune suppression protocols. Engraftment pattern 7 weeks after transplantation in mice treated with rapamycin combined with costimulation blockade with anti-CD40L and CTLA4 is shown in C and E. Typically, the developing spleen tissue consists of periarteriolar lymphoid sheath with mouse T cells (CD3, blue) and a mouse B cell zone (CD45R, green). The pig stroma is denoted in red by staining with anti-vimentin. A few mouse macrophages (green) are shown on the periphery of the follicle of chimeric spleen (E, long arrow). In contrast, the rejection pattern at 2 weeks after transplantation, shown in D and F (in the absence of immune suppression), shows no evidence of follicle structures. Thus, mouse T cells (blue) and B cells (green, shown by short arrow) are diffusely spread on residual pig stroma (D), and numerous mouse macrophages (F4/80, green) (F) are detected in the implant.
Similar results were described recently in this model for human and pig embryonic kidney (21) and for pig pancreatic precursor tissues (25).
Engraftment of E42 Pig Spleen Tissue in Immune-Competent Mice.
Although the E42 pig spleen tissue clearly exhibits reduced immunogenicity in the graft rejection model described above, this assay, because of the very low levels of human APC in the adoptively transferred PBMC, is largely biased toward detection of the direct immune rejection mechanism; however, rejection may also take place through the alternate indirect mechanism in which donor antigens are presented by host APCs to host T cells (cross-priming). Therefore, although the E42 implant is less immunogenic and is relatively less susceptible to direct recognition compared with E56 tissue, it could still be rejected via the indirect pathway in other murine models. Indeed, when transplanted in fully competent mice, embryonic pig spleen tissues of all gestational ages were promptly rejected (Fig. 3B).
To address this obstacle, we used an immune suppression protocol previously used in a mouse model of allogeneic skin transplantation. This protocol is based on a costimulatory blockade, inhibiting both CD28–B7 and CD40–CD40 ligand interactions, in conjunction with rapamycin, which is synergistic with these costimulatory agents (29).
As can be seen in Fig. 3B, engraftment and growth of E42 pig spleen precursor tissue were exhibited in recipient mice treated by this immune suppression protocol. In anticipation of problems that might be encountered with the implementation of daily rapamycin injections to hemophilic mice, we also attempted administration of rapamycin twice weekly, but this was associated with delayed rejection, observed by the fifth week after transplant (Fig. 3B).
Considering that, upon growth in SCID mice, pig embryonic spleen tissue was found to afford an adequate stroma for homing and expansion of mouse erythroid or myeloid cells, it was interesting to examine the presence of mouse lymphoid elements in growing pig spleens, when tested in fully immune-competent recipients, as opposed to SCID mice that lack T and B cells. To distinguish normal interaction and organization of mouse lymphoid follicles from potentially rejecting mouse T and B cells, the latter cell types were identified in proximity to the porcine stroma of the graft (vimentin 9) using triple staining for mouse T and B cells and for pig vimentin 9-positive cells. Indeed, similar to the architecture of adult spleen, in which the white pulp is organized as lymphoid sheaths, with T and B cell compartments around the branching arterial vessels, the pig E42 spleen tissue implanted under the optimal immune suppression protocol exhibited similar morphology and compartmentalization (Fig. 3C). In contrast, E42 spleen implants in C57BL/6 mice untreated by immune suppression showed spreading T cell patterns before complete rejection, and follicle structures were absent. (Fig. 3D). Likewise, staining of the host-type activated macrophages known to be mediating xenograft rejection revealed a distinctly different pattern in treated and untreated recipients (Fig. 3 E and F, respectively). Thus, in C57BL/6 mice treated with immune suppression, host macrophages did not invade the implants and could only be localized near T cell follicles, whereas in untreated mice both host macrophages and host T cells were diffusely distributed in the rejected graft.
Taken together, these results suggest that E42 pig precursor spleen tissues can grow and differentiate in fully immune-competent but immunosuppressed recipients and eventually develop a spleen-like structure.
Correction of Hemophilia by E42 Pig Spleen Tissue.
Based on several indications in the literature that factor VIII is produced in the spleen of adult tissue (14, 17, 18), we attempted to measure by RT-PCR the expression of pig factor VIII in the implants growing from embryonic spleen tissue obtained at different time points.
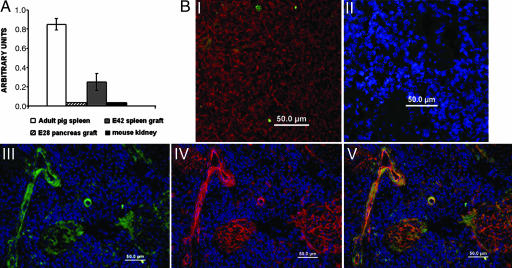
As can be seen in Fig. 4A, 6 weeks after transplantation of E42 spleen tissue, the grafts exhibited marked levels of porcine factor VIII mRNA expression, whereas pancreatic tissue growing out of embryonic pancreas precursor tissue did not exhibit an appreciable expression of factor VIII mRNA.
Fig. 4.
Expression of factor VIII in the growing E42 pig spleen implants. (A) Relative amounts of pig factor VIII mRNA in different transplanted tissues were evaluated by real-time PCR using pig factor VIII-specific primers. The results were normalized to the expression of the housekeeping gene, transferrin receptor. Pig factor VIII levels in E42 grafts are shown in gray. Total mRNA purified from adult pig spleen (white) and E28 pancreas graft (lines) served as positive and negative controls. Total mRNA purified from mouse kidney (black) was used to confirm factor VIII pig primer specificity. Data represent an average of three independent samples. (B) Immunolocalization of factor VIII. (BI) Expression of factor VIII in E42 spleen before transplantation reveals few positive cells (factor VIII is shown by green; vimentin is outlined in red), while preincubation of anti-factor VIII with factor VIII antigen (neutralization test) inhibits the staining (BII). Examination of the presence of factor VIII 3 months after implantation of E42 pig spleen into a factor VIII-KO SCID mouse is shown in BIII (green). Staining (red) of the same field with anti-pig CD31 (BIV) shows the presence of pig endothelial cells, and staining (BV) with both anti-factor VIII (green) and anti-pig CD31 (red) suggests colocalization in blood vessels (yellow).
Interestingly, staining of pig factor VIII by specific polyclonal antibodies (30) revealed that it is predominantly expressed in the E42 spleen implant by endothelial cells of pig origin. This conclusion is supported by double staining with anti-pig factor VIII and anti-pig CD31. Thus, as can be seen in Fig. 4B, the staining of factor VIII and CD31 is colocalized in blood vessels associated with the growing implant. These results are in line with similar indications that endothelial cells can serve as an important source of factor VIII (5, 31–33).
To ascertain the functionality of the transplanted tissue (i.e., factor VIII production), we initially attempted to treat factor VIII-KO mice (B6-129 background) by transplanting E42 pig spleen tissue using the immune suppression protocol of choice as previously described. However, although in humans oral delivery of rapamycin is straightforward and can be easily applied even to hemophilic patients, we found that in hemophilic mice the daily s.c. injection of any immune-suppressive agent is not practical because of bleeding complications.
Therefore, to circumvent the need for continuous s.c. injection of immune-suppressive agents, we developed a new strain of factor VIII-KO mice on a background of SCID mice, as previously described (34). We derived a colony of factor VIII-KO SCID mice, which were used to further analyze the potential of E42 spleen tissue to correct hemophilia. Indeed, transplantation in these mice, performed under short-term treatment with soluble factor VIII to enable the animals to tolerate the surgical procedure, was not associated with high mortality, and the mice were able to withstand repeated testing for factor VIII activity.
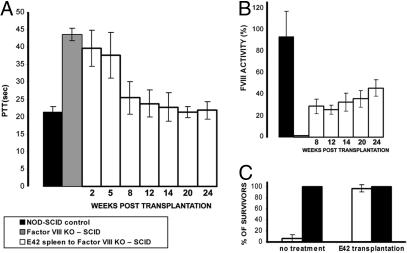
Traditionally, a clotting assay measuring partial thromboplastin time (PTT) is used for testing factor VIII activity. In addition, the indirect Coatest chromogenic assay offers a more sensitive determination of the presence of factor VIII by measuring its activity on cleavage of factor X. As can be seen in Fig. 5A, recipients of E42 spleen tissue exhibited normalized PTT levels by 2–3 months after transplant. Thus, while untreated factor VIII-KO SCID mice or those at 2 weeks after transplant exhibited PTT levels of 43.06 ± 1.07 sec and 39.63 ± 5.12 sec, respectively, the mice implanted with E42 spleen exhibited markedly reduced PTT values at 12 weeks after transplantation (23.4 ± 3.1 sec, P < 0.05), comparable with the levels found in control untreated, nonhemophilic SCID mice (21.23 ± 1.64 sec, P < 0.05).
Fig. 5.
Correction of hemophilia by implantation of pig E42 spleen tissue. (A) PTT values of wild-type NOD-SCID control mice (n = 10), factor VIII-KO SCID control mice (n = 10), and factor VIII-KO SCID mice (n = 37) transplanted with E42 pig spleen at different time intervals after transplantation. Data contain seven independent experiments. (B) Chromogenic determination of factor VIII activity in plasma of transplanted mice (n = 21). Comparison of factor VIII-KO SCID control mice (gray), wild-type NOD-SCID control mice (black), and factor VIII-KO SCID mice transplanted with E42 pig spleen at different time intervals after transplantation. (C) Survival after tail clipping of NOD-SCID control (black) and factor VIII-KO SCID (white) mice with and without E42 spleen transplantation. The data represent averages of three independent experiments (n > 5 in each experiment).
Likewise, factor VIII blood level determined by the Coatest assay (Fig. 5B) revealed significantly elevated levels in the blood of transplanted mice at 8 weeks after transplantation (28.6 ± 6. 8%) compared with the levels found in factor VIII-KO SCID mice (zero values) and in nonhemophilic SCID mice (93 ± 23.6%). Considering that severe, moderate, and mild hemophilic states, defined by a percentage of factor VIII, are below 1, 4, and 6%, respectively, it is significant that the levels in the entire group of transplanted mice range above these amounts (between 7% and 100% of factor VIII activity).
Finally, when exposed to tail clipping, 21 of 21 implanted mice survived, whereas almost all of the nonimplanted control mice died of bleeding within 24 h (Fig. 5C).
Taken together, our results show the feasibility of using embryonic spleen tissue, before the appearance of T cells in the tissue, for the correction of a genetic deficiency.
Discussion
Previous clinical attempts to correct monogenic diseases such as hemophilia A and Gaucher by allogeneic spleen transplantation were hampered by severe GVHD mediated by donor T cells present in the implanted spleen (35). Our current results suggest that transplantation of embryonic pig spleen precursor tissue, harvested before the appearance of GVHD-producing T cells in the spleen, namely at E42, leads to marked growth and differentiation affording a substantial source of tissue for correction of monogenic diseases.
The optimal growth of E42 pig spleen precursor tissue compared with earlier or later gestational time points was demonstrated by a quantitative morphometric analysis of tissue growth. In addition, the growing spleen tissue exhibited reduced immunogenicity compared with tissue growing from E56 or later time points. Interestingly, the stroma of the growing spleen tissue was found to accommodate hematopoietic host elements as well as the development of host B and T cell follicles resembling typical lymphoid structures in the normal adult spleen. Furthermore, RT-PCR and immunohistological staining show that factor VIII is predominantly expressed in endothelial cells in the growing spleen tissue. These data further support the early transplantation studies that suggested, indirectly, that the reticuloendothelial system seems to be the principle source of factor VIII (8, 15), and more recent data showing the role of liver sinusoidal endothelial cells (LSEC) in factor VIII secretion (5, 32).
Most relevant to our study is the demonstration of the ability of E42 pig spleen tissue to correct hemophilia A in factor VIII-KO SCID mice, providing a proof of principle for the potential of embryonic spleen tissue to afford a GVHD-free source for the correction of monogenic diseases.
Preliminary RT-PCR results suggest that the growing E42 spleen tissue does, in fact, exhibit significant expression not only of factor VIII but also of other relevant genes involved in Gaucher and other monogenic diseases.
An important issue that must be clarified in large animal models is the question of tissue dose, namely, whether more than one implant might be required to effectively correct hemophilia in large animals. Recent studies with pig embryonic pancreatic precursor tissue suggest that several tissues from different embryos can be pooled so as to provide a more effective therapy of diabetes (S.E.-F., E.S., and G. Heacht, unpublished work).
Another major obstacle to the implementation of embryonic allotransplantation or xenotransplantation in patients is related to its potential immunogenicity.
Considering that allogeneic or xenogenic embryonic spleen transplants will require some form of immune suppression, which is associated with some risks, it is envisioned that this approach will be most suitable in diseases for which no therapy is currently available. Thus, although we demonstrated the proof of principle in hemophilic mice, the clinical evaluation of this approach might initially be more justified in other diseases, for which replacement therapy with an exogenous enzyme or factor is not available.
Another major issue that must be addressed in further studies is related to the choice of fetal tissue. As a proof of principle, we chose to evaluate the potential of pig embryonic tissue. Several safety concerns, in particular the potential hazards associated with porcine endogenous retroviruses (PERV), have presented major obstacles for this application (40–42). However, it is important to note that previous pig-to-human xenotransplantations have failed to reveal even a single instance of PERV transmission to a human subject (43). Moreover, a recent study has suggested that PERV could be eradicated from pig herds bred for xenotransplantation (44). Thus, as suggested recently by Ogata and Platt (45), although the potential danger of PERV to public health cannot be entirely dismissed, it should be approached with careful attention to the xenograft recipients, rather than necessitating that xenotransplantation studies be abandoned. Indeed, National Institutes of Health and Food and Drug Administration guidelines for xenogenic transplants are in line with this view. However, pig embryonic tissue, while circumventing ethical issues associated with human embryonic stem cells or tissues, might be more prone to rejection compared with human fetal spleen tissue.
Therefore, the use of human fetal spleen tissue from early abortions, if deemed ethically acceptable, might represent a potentially superior tissue source.
Our results showing that the growing spleen stroma can support hematopoiesis and afford a potentially new lymphoid site with host T and B cell follicles indicate that embryonic allogeneic spleen tissue could also be used to enhance hematopoietic or immune reconstitution in different pathological situations. Optimal gestational time for harvesting human spleen tissue is currently under investigation in SCID mice.
In conclusion, regardless of the immune suppression modalities that will be required and must be defined in large animal models, our data provide a proof of principle for the curative potential of T cell-free, embryonic spleen tissue as a novel source for transplantation in patients with genetic deficiencies. This approach could be especially valuable in the treatment of diseases for which no therapy is currently available.
Materials and Methods
Animals.
Animals were maintained under conditions approved by the Institutional Animal Care and Use Committee of the Weizmann Institute. A breeding pair of factor VIII-KO mice was purchased from The Jackson Laboratory (strain name B6;129S4-F8tm1Kaz/J; stock no. 004424).
To obtain immunodeficient hemophilic mice (designated as factor VIII-KO SCID), factor VIII-deficient mice were crossed with SCID mice. Genotyping and phenotypic characterization of the factor VIII-KO and factor VIII-KO SCID offspring were performed, confirming that all factor VIII-deficient mice used in this study are factor VIII-KO SCID mice.
C57BL/6, immune-deficient NOD-SCID, or factor VIII-KO SCID mice (The Weizmann Institute Animal Breeding Center) were used as hosts for the transplantation studies at the age of 8–10 weeks. All mice were kept in small cages (up to five animals in each cage) and fed sterile food and acid water containing ciprofloxacin (20 mg/ml).
Pig embryos were obtained from the Lahav Institute of Animal Research (Kibbutz Lahav, Israel). Pregnant sows were operated on at specific stages of the pregnancy (E24, E28, E42, E56, E80, and E100) under general anesthesia. Warm ischemia time was <10 min, and the embryos were transferred to cold PBS. Spleen precursors for transplantation were extracted under a light microscope and were kept in sterile conditions at 4°C in RPMI medium 1640 (Biological Industries) before transplantation. Cold ischemia time until transplantation was <2 h. The study protocol was approved by the ethics committees at Kibbutz Lahav and the Weizmann Institute.
Transplantation Procedure.
Transplantations of the embryonic precursors were performed under general anesthesia (2.5% 2,2,2-tribromoethanol, 97% in PBS, 10 ml/kg, i.p.). Host kidney was exposed through a left lateral incision. A 1.5-mm incision was made at the caudal end of the kidney capsule, and donor precursors were grafted under the kidney capsule in fragments 1–2 mm in diameter.
Isolation and Transfer of Human PBMC.
Human PBMC were generated from two portions of buffy coats obtained from the Israeli Blood Bank (Chaim Sheba Medical Center, Tel Hashomer, Israel). Cells were fractionated on Ficoll (Amersham Biosciences). Human cells (80 × 106) were injected once i.p. into SCID mice, 1–3 days after the pig spleen precursor's transplantation. Control mice did not receive human PBMC. For analysis of human lymphocyte engraftment, we measured human IgG in the mouse serum by standard ELISA procedure, and positive mice were further analyzed.
Plasma Factor VIII Assays.
Factor VIII activity was assayed in citrated plasma collected from recipients. PTT was determined with a coagulometer Sysmex CA-6000. (Assays were performed in the clinical hematology laboratory of Kaplan Medical Center, Rehovot.) The chromogenic activity assay, which measures the factor VIII-dependent cleavage of factor Xa from factor X (COATEST FVIII; Chromogenix) was performed.
Tail Clipping.
Tail clipping (≈1.5 cm from the tip) was performed without subsequent cauterization to measure bleeding propensity. After the procedure, mice were checked every 4 h. The proportion of surviving mice at 24 h after the procedure was recorded.
Statistical Analysis.
Comparisons between groups were evaluated by using Student's t test. Data were expressed as mean ± SD and were considered statistically significant at P ≤ 0.05.
Supporting Information.
For further details regarding morphometric analysis, immunohistochemistry, and read-time PCR, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Dr. Jan. A. van Mourik for his generous gift of anti-pig factor VIII antibody. This work was supported by Tissera, Inc.
Abbreviations
- En
embryonic day n
- GVHD
graft versus host disease
- PBMC
peripheral blood mononuclear cell
- PTT
partial thromboplastin time.
Footnotes
Conflict of interest statement: Y.R. serves as the Chairman of the Advisory Board of and holds equity and has patent arrangements with Tissera, Inc., which supported this work.
This article is a PNAS direct submission.
References
- 1.Nabel GJ. Nat Med. 2004;10:135–141. doi: 10.1038/nm990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connelly S, Andrews JL, Gallo AM, Kayda DB, Qian J, Hoyer L, Kadan MJ, Gorziglia MI, Trapnell BC, McClelland A, Kaleko M. Blood. 1998;91:3273–3281. [PubMed] [Google Scholar]
- 3.Tiede A, Eder M, von Depka M, Battmer K, Luther S, Kiem HP, Ganser A, Scherr M. Gene Ther. 2003;10:1917–1925. doi: 10.1038/sj.gt.3302093. [DOI] [PubMed] [Google Scholar]
- 4.Van Damme A, Chuah MK, Dell'accio F, De Bari C, Luyten F, Collen D, Vanden Driessche T. Haemophilia. 2003;9:94–103. doi: 10.1046/j.1365-2516.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 5.Do H, Healey JF, Waller EK, Lollar P. J Biol Chem. 1999;274:19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- 6.Ashrani AA, Reding MT, Shet A, Osip J, Humar A, Lake JR, Key NS. Haemophilia. 2004;10:735–737. doi: 10.1111/j.1365-2516.2004.01030.x. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JH, Bontempo FA, Spero JA, Ragni MV, Starzl TE. N Engl J Med. 1985;312:1189–1190. doi: 10.1056/NEJM198505023121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchioro TL, Hougie C, Ragde H, Epstein RB, Thomas ED. Science. 1969;163:188–190. doi: 10.1126/science.163.3863.188. [DOI] [PubMed] [Google Scholar]
- 9.Gordon FH, Mistry PK, Sabin CA, Lee CA. Gut. 1998;42:744–749. doi: 10.1136/gut.42.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko S, Tanaka I, Kanokogi H, Kanehiro H, Okayama J, Ori J, Shima M, Yoshioka A, Giles A, Nakajima Y. Transplant Proc. 2005;37:1131–1133. doi: 10.1016/j.transproceed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Moore FD, Wheele HB, Demissianos HV, Smith LL, Balankura O, Abel K, Greenberg JB, Dammin GJ. Ann Surg. 1960;152:374–387. [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchioro TL, Rowlands DT, Jr, Rifkind D, Waddell WR, Starzl TE, Fudenberg H. Ann NY Acad Sci. 1964;120:626–651. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hathaway WE, Mull MM, Githens JH, Groth CG, Marchioro TL, Starzl TE. Transplantation. 1969;7:73–75. doi: 10.1097/00007890-196901000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groth CG, Hathaway WE, Gustafsson A, Geis WP, Putnam CW, Bjorken C, Porter KA, Starzl TE. Surgery. 1974;75:725–733. [PMC free article] [PubMed] [Google Scholar]
- 16.Veltkamp JJ, Asfaou E, van de Torren K, van der Does JA, van Tilburg NH, Pauwels EK. Transplantation. 1974;18:56–62. doi: 10.1097/00007890-197407000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Xiang WZ, Jie ZW, Sheng XS. Transplant Proc. 2002;34:1929–1931. doi: 10.1016/s0041-1345(02)03127-5. [DOI] [PubMed] [Google Scholar]
- 18.Dor FJ, Gollackner B, Cooper DK. Transplant Int. 2003;16:451–460. doi: 10.1007/s00147-003-0640-0. [DOI] [PubMed] [Google Scholar]
- 19.Groth CG, Hagenfeldt L, Dreborg S, Lofstrom B, Ockerman PA, Samuelsson K, Svennerholm L, Werner B, Westberg G. Lancet. 1971;1:1260–1264. doi: 10.1016/s0140-6736(71)91778-8. [DOI] [PubMed] [Google Scholar]
- 20.Pappworth MH. Lancet. 1971;2:220. doi: 10.1016/s0140-6736(71)90937-8. [DOI] [PubMed] [Google Scholar]
- 21.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH, Reisner Y. Nat Med. 2003;9:53–60. doi: 10.1038/nm812. [DOI] [PubMed] [Google Scholar]
- 22.Eventov-Friedman S, Katchman H, Shezen E, Aronovich A, Tchorsh D, Dekel B, Freud E, Reisner Y. Proc Natl Acad Sci USA. 2005;102:2928–2933. doi: 10.1073/pnas.0500177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohn W, Wiegers W, Beuttenmuller M, Traub P. Exp Cell Res. 1992;201:1–7. doi: 10.1016/0014-4827(92)90341-5. [DOI] [PubMed] [Google Scholar]
- 24.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 25.Eventov-Friedman S, Tchorsh D, Katchman H, Shezen E, Aronovich A, Hecht G, Dekel B, Rechavi G, Blazar BR, Feine I, et al. PLoS Med. 2006;3:e215. doi: 10.1371/journal.pmed.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekel B, Burakova T, Marcus H, Shezen E, Polack S, Canaan A, Passwell J, Reisner Y. Transplantation. 1997;64:1541–1550. doi: 10.1097/00007890-199712150-00007. [DOI] [PubMed] [Google Scholar]
- 27.Dekel B, Burakova T, Ben-Hur H, Marcus H, Oren R, Laufer J, Reisner Y. Transplantation. 1997;64:1550–1558. doi: 10.1097/00007890-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Lubin I, Segall H, Marcus H, David M, Kulova L, Steinitz M, Erlich P, Gan J, Reisner Y. Blood. 1994;83:2368–2381. [PubMed] [Google Scholar]
- 29.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 30.Hollestelle MJ, Poyck PP, Hollestelle JM, Marsman HA, Mourik JA, Gulik TM. J Thromb Haemost. 2005;3:2274–2280. doi: 10.1111/j.1538-7836.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Nichols TC, Sarkar R, McCorquodale S, Bellinger DA, Ponder KP. Proc Natl Acad Sci USA. 2005;102:6080–6085. doi: 10.1073/pnas.0409249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumaran V, Benten D, Follenzi A, Joseph B, Sarkar R, Gupta S. J Thromb Haemost. 2005;3:2022–2031. doi: 10.1111/j.1538-7836.2005.01508.x. [DOI] [PubMed] [Google Scholar]
- 33.Hollestelle MJ, Thinnes T, Crain K, Stiko A, Kruijt JK, van Berkel TJ, Loskutoff DJ, van Mourik JA. Thromb Haemost. 2001;86:855–861. [PubMed] [Google Scholar]
- 34.Chuah MK, Schiedner G, Thorrez L, Brown B, Johnston M, Gillijns V, Hertel S, Van Rooijen N, Lillicrap D, Collen D, et al. Blood. 2003;101:1734–1743. doi: 10.1182/blood-2002-03-0823. [DOI] [PubMed] [Google Scholar]
- 35.Xia SS, Jiang HC, Zhou XX, He G. Chin Med J (Engl) 1992;105:609–611. [PubMed] [Google Scholar]
- 36.Medawar PB. Symp Soc Exp Biol. 1953;7:320–323. [Google Scholar]
- 37.Erdag G, Morgan JR. Transplantation. 2002;73:519–528. doi: 10.1097/00007890-200202270-00005. [DOI] [PubMed] [Google Scholar]
- 38.Foglia RP, LaQuaglia M, DiPreta J, Donahoe PK. J Pediatr Surg. 1986;21:608–612. doi: 10.1016/s0022-3468(86)80415-8. [DOI] [PubMed] [Google Scholar]
- 39.Foglia RP, DiPreta J, Statter MB, Donahoe PK. Ann Surg. 1986;204:402–410. doi: 10.1097/00000658-198610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langford GA, Galbraith D, Whittam AJ, McEwan P, Fernandez-Suarez XM, Black J, Shepherd A, Onions D. Transplantation. 2001;72:1996–2000. doi: 10.1097/00007890-200112270-00023. [DOI] [PubMed] [Google Scholar]
- 41.Tacke SJ, Bodusch K, Berg A, Denner J. Xenotransplantation. 2001;8:125–135. [PubMed] [Google Scholar]
- 42.Elliott RB, Escobar L, Garkavenko O, Croxson MC, Schroeder BA, McGregor M, Ferguson G, Beckman N, Ferguson S. Cell Transplant. 2000;9:895–901. doi: 10.1177/096368970000900616. [DOI] [PubMed] [Google Scholar]
- 43.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, Otto E. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 44.Clark DA, Fryer JF, Tucker AW, McArdle PD, Hughes AE, Emery VC, Griffiths PD. Xenotransplantation. 2003;10:142–148. doi: 10.1034/j.1399-3089.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- 45.Ogata K, Platt JL. J Heart Lung Transplant. 2004;23:515–526. doi: 10.1016/j.healun.2003.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.