In the 18th Century, the Swedish botanist Karl von Linné designed a ‘Flower-Clock' by arranging a series of various plant species according to the respective time their flowers open or close every day. Watching this ‘Flower-Clock', one can then estimate the time of the day by noting the pattern of flower opening and closing. It has been a well-known fact since Linné's early times that plants can open or close their flowers at a precise time of the day. However, we still do not fully understand the design principles of the molecular network that underlies the cellular circadian clock, which achieves to predict, often with exquisite precision, the cyclic changes in the environment due to the rotation of earth. In two articles currently published in Molecular Systems Biology, Millar and co-workers (Locke et al, 2006) and Doyle and co-workers (Zeilinger et al, 2006) propose a plausible design for the plant circadian clock.
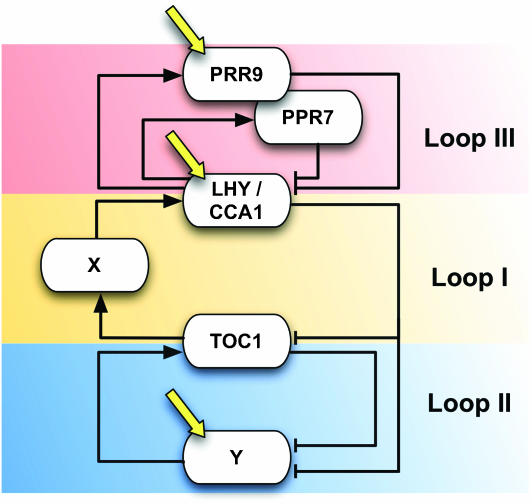
In previous work, Millar and co-workers extended an initial ‘one-loop model' of the plant circadian clock into a ‘two-loop model' (Figure 1) (Locke et al, 2005). In the simple ‘one-loop model' (Figure 1, loop I), two partially redundant genes, LATE ELONGATED HYPOCOTYL (LHY) and CIRCADIAN CLOCK ASSOCIATED 1 (CCA1), repress the expression of their activator, TIMING OF CAB EXPRESSION 1 (TOC1) (Alabadi et al, 2001). In this model, light activates the expression of LHY/CCA1, according to experimental data that show a response of LHY and CCA1 transcription to light stimulation (Wang and Tobin, 1998; Martinez-Garcia et al, 2000; Kim et al, 2003). The simple ‘one-loop model' cannot explain some experimental data, such as the short period rhythm in lhy;cca1 mutants (Alabadi et al, 2002; Locke et al, 2005). In order to explain the residual rhythm in lhy;cca1 plants, Millar and co-workers incorporated two hypothetical components, X and Y, to develop a ‘two-loop model' (Figure 1, loops I and II). In this extended model, TOC1 is proposed to activate the expression of X, which, in turn, activates LHY/CCA1 transcription, as required by the time-course profile of TOC1 protein (Mas et al, 2003b). The second loop is formed by Y and TOC1, and is responsible for the short-period oscillation in the lhy;cca1 mutant. Y is also proposed to be activated by light, because TOC1 transcription has been shown to respond to light, although there is no evidence of direct light activation of TOC1 transcription (Makino et al, 2001). Although the ‘two-loop model' can explain many aspects of plant circadian clocks (Locke et al, 2005), it still cannot explain some experimental data, including the residual short-period rhythm observed in the toc1 mutants (Mas et al, 2003a) and the very long-period rhythm of double mutants for PSEUDO-RESPONSE REGULATOR 7 (PRR7) and PRR9 (Farre et al, 2005).
Figure 1.
Schematic representation of the proposed models of the plant circadian clock. X and Y are hypothetical proteins. Yellow arrows indicate light input.
In order to explain the latter experimental results, Millar and co-workers (Locke et al, 2006) and Doyle and co-workers (Zeilinger et al, 2006) incorporated the recently proposed feedback loop between PRR7/PRR9 and LHY/CCA1 (Farre et al, 2005; Salome and McClung, 2005) and proposed a further extension of the model into a ‘three-loop model' (Figure 1, loops I–III). In this new model, PRR7/PRR9 are proposed to be activated by LHY/CCA1, although PRR7/PRR9 proteins repress LHY/CCA1 transcription. Light activates the expression of PRR7/PRR9 in Millar's study, or PRR9 in Doyle's model, as PRR9 has been shown to be acutely activated by light (Ito et al, 2003). Millar's and Doyle's models are very similar in their global structure, but differ slightly in how light induction of Y and LHY/CCA1 is modeled, and in the details of the PRR7/PRR9-LHY/CCA1 loop mechanism. For example, Millar's model assumes that light induction of Y and LHY/CCA1 depends on both a continuous and a transient mechanism, whereas Doyle and co-workers propose a more sophisticated mechanism, whereby light induction of Y is dependent on a continuous mechanism, whereas that of LHY/CCA1 is dependent on a transient mechanism. For the purpose of simplification, PRR7/PRR9 are dealt as one factor in Millar's work, whereas PRR7 and PRR9 are more realistically treated as two different factors in Doyle's model. It is also noteworthy that Millar and co-workers analyzed the rhythms of gi;lhy;cca1 triple mutant plants to further experimentally validate their proposal that GI is a strong candidate for being part of the hypothetical Y component (Locke et al, 2006), and that Doyle's and co-workers performed a detailed sensitivity analysis to identify the points of strength and weakness in the current ‘three-loop model', thus providing a guide for future experimental and modeling efforts (Zeilinger et al, 2006).
In both cases, the ‘three-loop model' suggests an interesting design principle underlying the plant clock. The morning oscillator, PRR7/PRR9-LHY/CCA1 loop (Figure 1, loop III), and the evening oscillator, TOC1-Y loop (Figure 1, loop II), are coupled together via the LHY/CCA1-TOC1-X loop (Figure 1, loop I). These coupled morning and evening oscillators may provide the flexibility to track dawn and dusk and, thus, confer the clock with the capability of measuring the length of the day (or intervals of multiple phases) under conditions of changing photoperiods. In order to formally prove the proposed ‘three-loop model', it will be necessary to uncover the identity of the missing factor X linking the morning and evening oscillators. Only time will tell how plausible biologically significant the ‘three-loop model' really is, but the perspective that an X mutation will cause the morning and evening oscillators to run with different periods within the same cell is surely an exciting one. We can only hope that such a discovery will be reported in the near future!
References
- Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883 [DOI] [PubMed] [Google Scholar]
- Alabadi D, Yanovsky MJ, Mas P, Harmer SL, Kay SA (2002) Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr Biol 12: 757–761 [DOI] [PubMed] [Google Scholar]
- Farre EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA (2005) Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr Biol 15: 47–54 [DOI] [PubMed] [Google Scholar]
- Ito S, Matsushika A, Yamada H, Sato S, Kato T, Tabata S, Yamashino T, Mizuno T (2003) Characterization of the APRR9 pseudo-response regulator belonging to the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol 44: 1237–1245 [DOI] [PubMed] [Google Scholar]
- Kim JY, Song HR, Taylor BL, Carre IA (2003) Light-regulated translation mediates gated induction of the Arabidopsis clock protein LHY. EMBO J 22: 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JCW, Kozma-Bognar L, Gould PD, Fehér B, Kevei É, Nagy F, Turner MS, Hall A, Millar AJ (2006) Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol Syst Biol 2: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JCW, Southern MM, Kozma-Bognar L, Hibberd V, Brown PE, Turner MS, Millar AJ (2005) Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol Syst Biol 1: 2005.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Matsushika A, Kojima M, Oda Y, Mizuno T (2001) Light response of the circadian waves of the APRR1/TOC1 quintet: when does the quintet start singing rhythmically in Arabidopsis? Plant Cell Physiol 42: 334–339 [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia JF, Huq E, Quail PH (2000) Direct targeting of light signals to a promoter element-bound transcription factor. Science 288: 859–863 [DOI] [PubMed] [Google Scholar]
- Mas P, Alabadi D, Yanovsky MJ, Oyama T, Kay SA (2003a) Dual role of TOC1 in the control of circadian and photomorphogenic responses in Arabidopsis. Plant Cell 15: 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas P, Kim WY, Somers DE, Kay SA (2003b) Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426: 567–570 [DOI] [PubMed] [Google Scholar]
- Salome PA, McClung CR (2005) Pseudo-response regulator 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell 17: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Tobin EM (1998) Constitutive expression of the circadian clock associated 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93: 1207–1217 [DOI] [PubMed] [Google Scholar]
- Zeilinger MN, Farré EM, Taylor SR, Kay SA, Doyle III FJ (2006) A novel computational model of the circadian clock in Arabidopsis that incorporates PRR7 and PRR9. Mol Syst Biol 2: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]