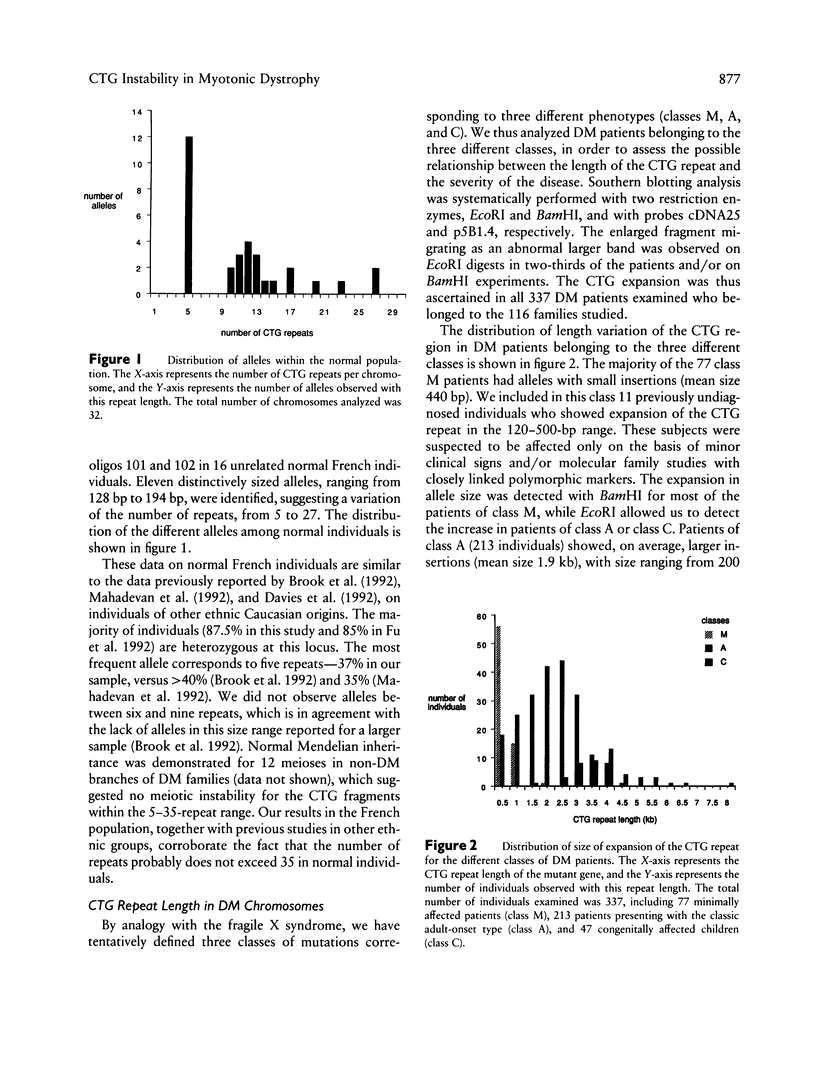
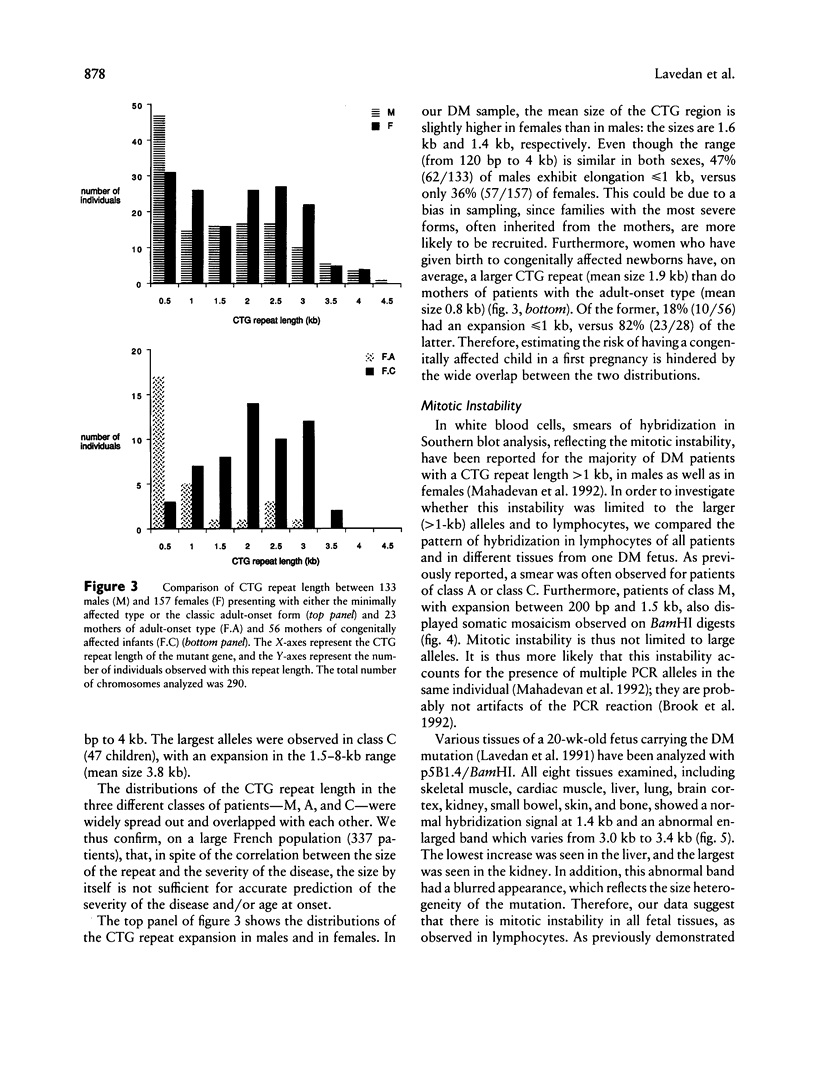
Abstract
Myotonic dystrophy (DM) is a progressive neuromuscular disorder which results from elongations of an unstable (CTG)n repeat, located in the 3' untranslated region of the DM gene. A correlation has been demonstrated between the increase in the repeat number of this sequence and the severity of the disease. However, the clinical status of patients cannot be unambiguously ascertained solely on the basis of the number of CTG repeats. Moreover, the exclusive maternal inheritance of the congenital form remains unexplained. Our observation of differently sized repeats in various DM tissues from the same individual may explain why the size of the mutation observed in lymphocytes does not necessarily correlate with the severity and nature of symptoms. Through a molecular and genetic study of 142 families including 418 DM patients, we have investigated the dynamics of the CTG repeat meiotic instability. A positive correlation between the size of the repeat and the intergenerational enlargement was observed similarly through male and female meioses for < or = 0.5-kb CTG sequences. Beyond 0.5 kb, the intergenerational variation was more important through female meioses, whereas a tendency to compression was observed almost exclusively in male meioses, for > or = 1.5-kb fragments. This implies a size- and sex-dependent meiotic instability. Moreover, segregation analysis supports the hypothesis of a maternal as well as a familial predisposition for the occurrence of the congenital form. Finally, this analysis reveals a significant excess of transmitting grandfathers partially accounted for by increased fertility in affected males.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992 Feb 6;355(6360):548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- Brook J. D., Harley H. G., Walsh K. V., Rundle S. A., Siciliano M. J., Harper P. S., Shaw D. J. Identification of new DNA markers close to the myotonic dystrophy locus. J Med Genet. 1991 Feb;28(2):84–88. doi: 10.1136/jmg.28.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Buxton J., Shelbourne P., Davies J., Jones C., Van Tongeren T., Aslanidis C., de Jong P., Jansen G., Anvret M., Riley B. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature. 1992 Feb 6;355(6360):547–548. doi: 10.1038/355547a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Pizzuti A., Fu Y. H., Fenwick R. G., Jr, Nelson D. L. Triplet repeat mutations in human disease. Science. 1992 May 8;256(5058):784–789. doi: 10.1126/science.1589758. [DOI] [PubMed] [Google Scholar]
- Davies J., Yamagata H., Shelbourne P., Buxton J., Ogihara T., Nokelainen P., Nakagawa M., Williamson R., Johnson K., Miki T. Comparison of the myotonic dystrophy associated CTG repeat in European and Japanese populations. J Med Genet. 1992 Nov;29(11):766–769. doi: 10.1136/jmg.29.11.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devys D., Biancalana V., Rousseau F., Boué J., Mandel J. L., Oberlé I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. 1992 Apr 15-May 1Am J Med Genet. 43(1-2):208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Pizzuti A., Fenwick R. G., Jr, King J., Rajnarayan S., Dunne P. W., Dubel J., Nasser G. A., Ashizawa T., de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992 Mar 6;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Floyd J., Rundle S. A., Crow S., Walsh K. V., Thibault M. C., Harper P. S., Shaw D. J. Detection of linkage disequilibrium between the myotonic dystrophy locus and a new polymorphic DNA marker. Am J Hum Genet. 1991 Jul;49(1):68–75. [PMC free article] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Rundle S. A., Crow S., Reardon W., Buckler A. J., Harper P. S., Housman D. E., Shaw D. J. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992 Feb 6;355(6360):545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Rundle S. A., Reardon W., Myring J., Crow S., Brook J. D., Harper P. S., Shaw D. J. Unstable DNA sequence in myotonic dystrophy. Lancet. 1992 May 9;339(8802):1125–1128. doi: 10.1016/0140-6736(92)90729-m. [DOI] [PubMed] [Google Scholar]
- Harper P. S., Harley H. G., Reardon W., Shaw D. J. Anticipation in myotonic dystrophy: new light on an old problem. Am J Hum Genet. 1992 Jul;51(1):10–16. [PMC free article] [PubMed] [Google Scholar]
- Henry I., Uzan G., Weil D., Nicolas H., Kaplan J. C., Marguerie C., Kahn A., Junien C. The genes coding for A alpha-, B beta-, and gamma-chains of fibrinogen map to 4q2. Am J Hum Genet. 1984 Jul;36(4):760–768. [PMC free article] [PubMed] [Google Scholar]
- Koch M. C., Grimm T., Harley H. G., Harper P. S. Genetic risks for children of women with myotonic dystrophy. Am J Hum Genet. 1991 Jun;48(6):1084–1091. [PMC free article] [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Morton N. E., Macpherson J. N. Population genetics of the fragile-X syndrome: multiallelic model for the FMR1 locus. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4215–4217. doi: 10.1073/pnas.89.9.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelbourne P., Winqvist R., Kunert E., Davies J., Leisti J., Thiele H., Bachmann H., Buxton J., Williamson B., Johnson K. Unstable DNA may be responsible for the incomplete penetrance of the myotonic dystrophy phenotype. Hum Mol Genet. 1992 Oct;1(7):467–473. doi: 10.1093/hmg/1.7.467. [DOI] [PubMed] [Google Scholar]
- Tsilfidis C., MacKenzie A. E., Mettler G., Barceló J., Korneluk R. G. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat Genet. 1992 Jun;1(3):192–195. doi: 10.1038/ng0692-192. [DOI] [PubMed] [Google Scholar]
- VANIER T. M. Dystrophia myotonica in childhood. Br Med J. 1960 Oct 29;2(5208):1284–1288. doi: 10.1136/bmj.2.5208.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Miki T., Ogihara T., Nakagawa M., Higuchi I., Osame M., Shelbourne P., Davies J., Johnson K. Expansion of unstable DNA region in Japanese myotonic dystrophy patients. Lancet. 1992 Mar 14;339(8794):692–692. doi: 10.1016/0140-6736(92)90862-w. [DOI] [PubMed] [Google Scholar]