Abstract
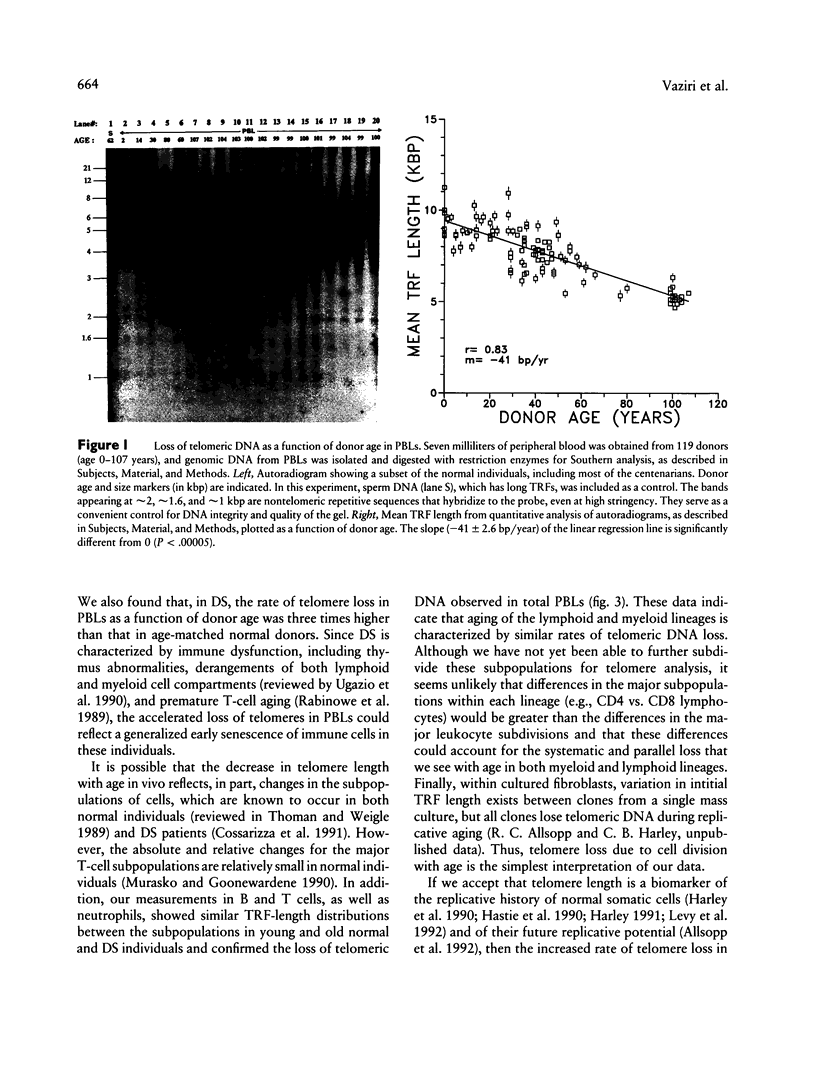
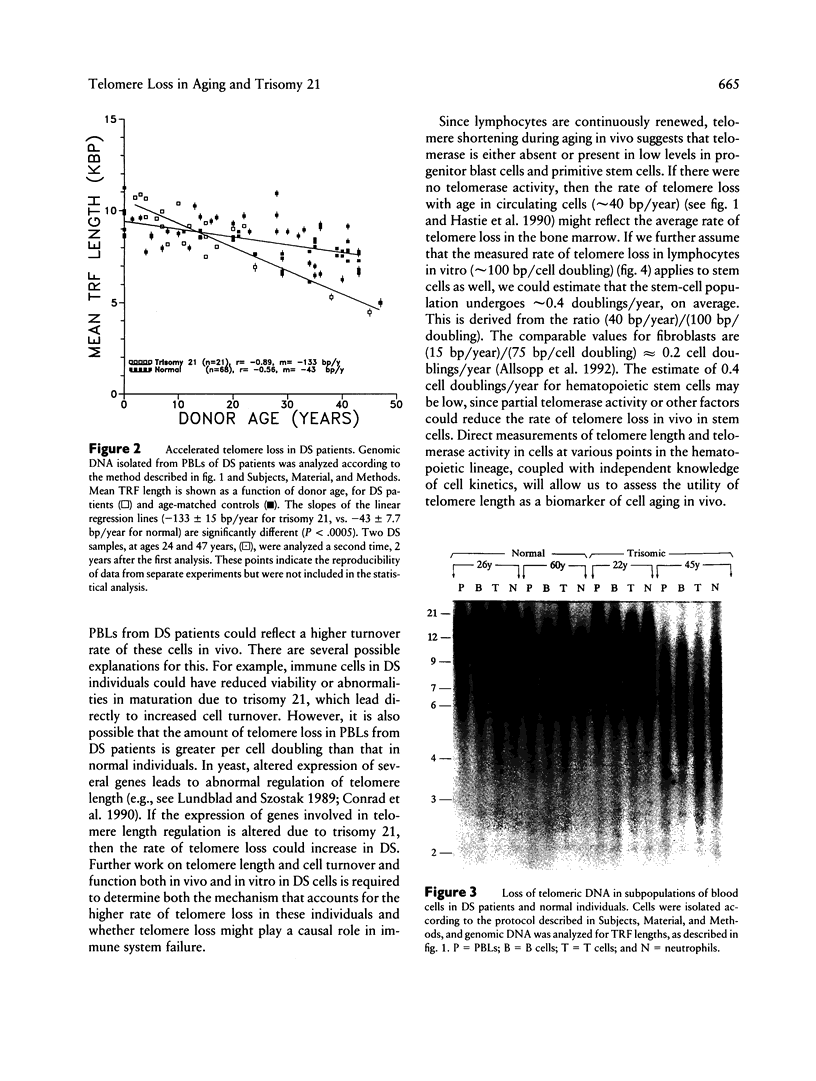
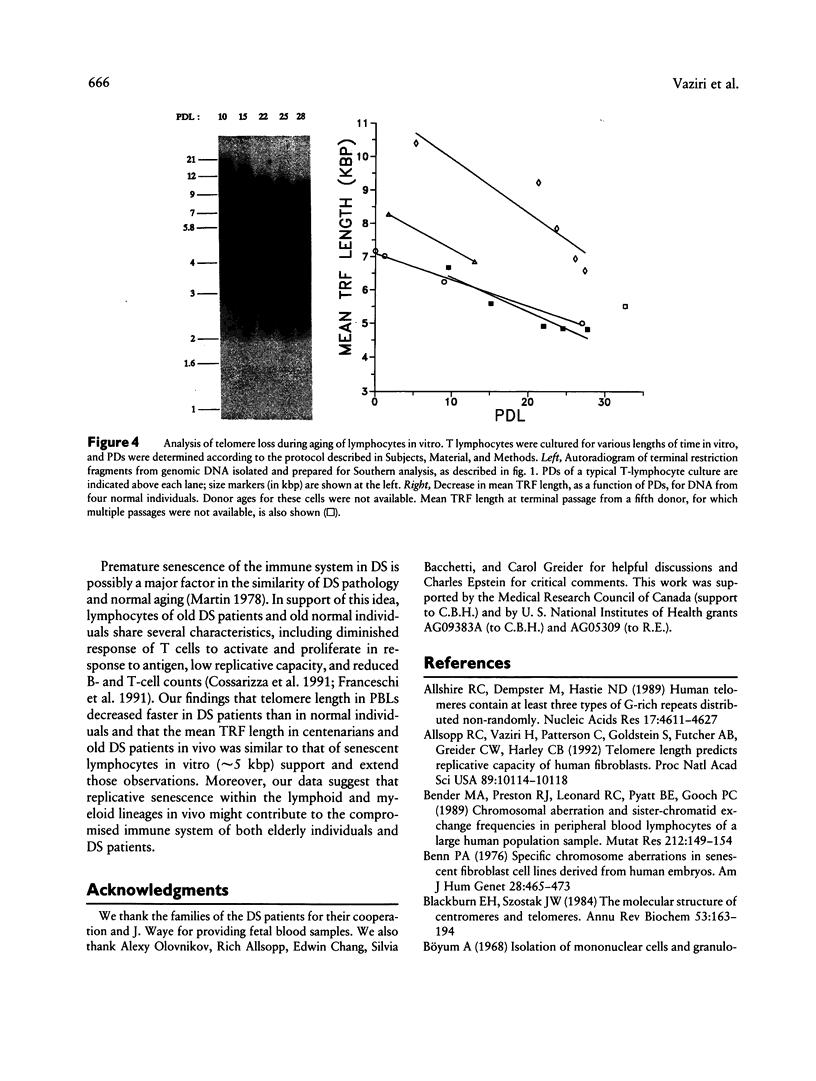
The telomere hypothesis of cellular aging proposes that loss of telomeric DNA (TTAGGG) from human chromosomes may ultimately cause cell-cycle exit during replicative senescence. Since lymphocytes have a limited replicative capacity and since blood cells were previously shown to lose telomeric DNA during aging in vivo, we wished to determine: (a) whether accelerated telomere loss is associated with the premature immunosenescence of lymphocytes in individuals with Down syndrome (DS) and (b) whether telomeric DNA is also lost during aging of lymphocytes in vitro. To investigate the effects of aging and trisomy 21 on telomere loss in vivo, genomic DNA was isolated from peripheral blood lymphocytes of 140 individuals (age 0-107 years), including 21 DS patients (age 0-45 years). Digestion with restriction enzymes HinfI and RsaI generated terminal restriction fragments (TRFs), which were detected by Southern analysis using a telomere-specific probe (32P-(C3TA2)3). The rate of telomere loss was calculated from the decrease in mean TRF length, as a function of donor age. DS patients showed a significantly higher rate of telomere loss with donor age (133 +/- 15 bp/year) compared with age-matched controls (41 +/- 7.7 bp/year) (P < .0005), suggesting that accelerated telomere loss is a biomarker of premature immunosenescence of DS patients and that it may play a role in this process. Telomere loss during aging in vitro was calculated for lymphocytes from four normal individuals, grown in culture for 10-30 population doublings. The rate of telomere loss was approximately 120 bp/cell doubling, comparable to that seen in other somatic cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Dempster M., Hastie N. D. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989 Jun 26;17(12):4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Preston R. J., Leonard R. C., Pyatt B. E., Gooch P. C. Chromosomal aberration and sister-chromatid exchange frequencies in peripheral blood lymphocytes of a large human population sample. II. Extension of age range. Mutat Res. 1989 Jun;212(2):149–154. doi: 10.1016/0027-5107(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Benn P. A. Specific chromosome aberrations in senescent fibroblast cell lines derived from human embryos. Am J Hum Genet. 1976 Sep;28(5):465–473. [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Conrad M. N., Wright J. H., Wolf A. J., Zakian V. A. RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell. 1990 Nov 16;63(4):739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Cossarizza A., Ortolani C., Forti E., Montagnani G., Paganelli R., Zannotti M., Marini M., Monti D., Franceschi C. Age-related expansion of functionally inefficient cells with markers of natural killer activity in Down's syndrome. Blood. 1991 Mar 15;77(6):1263–1270. [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley D. C., Simpson J. F., Meryman H. T. Isolation of large numbers of fully viable human neutrophils: a preparative technique using percoll density gradient centrifugation. Exp Hematol. 1982 Aug;10(7):591–599. [PubMed] [Google Scholar]
- Franceschi C., Monti D., Cossarizza A., Fagnoni F., Passeri G., Sansoni P. Aging, longevity, and cancer: studies in Down's syndrome and centenarians. Ann N Y Acad Sci. 1991;621:428–440. doi: 10.1111/j.1749-6632.1991.tb16997.x. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Bernabe R. R., Vega J., Kreisler M. Purification of human T and B cells by a discontinuous density gradient of percoll. J Immunol Methods. 1979;29(1):57–63. doi: 10.1016/0022-1759(79)90125-x. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Levy M. Z., Allsopp R. C., Futcher A. B., Greider C. W., Harley C. B. Telomere end-replication problem and cell aging. J Mol Biol. 1992 Jun 20;225(4):951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Szostak J. W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989 May 19;57(4):633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Martin G. M. Genetic syndromes in man with potential relevance to the pathobiology of aging. Birth Defects Orig Artic Ser. 1978;14(1):5–39. [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasko D. M., Goonewardene I. M. T-cell function in aging: mechanisms of decline. Annu Rev Gerontol Geriatr. 1990;10:71–96. doi: 10.1007/978-3-662-38445-9_5. [DOI] [PubMed] [Google Scholar]
- Olovnikov A. M. Printsip marginotomii v matrichnom sinteze polinukleotidov. Dokl Akad Nauk SSSR. 1971;201(6):1496–1499. [PubMed] [Google Scholar]
- Rabinowe S. L., Rubin I. L., George K. L., Adri M. N., Eisenbarth G. S. Trisomy 21 (Down's syndrome): autoimmunity, aging and monoclonal antibody-defined T-cell abnormalities. J Autoimmun. 1989 Feb;2(1):25–30. doi: 10.1016/0896-8411(89)90105-4. [DOI] [PubMed] [Google Scholar]
- SAKSELA E., MOORHEAD P. S. ANEUPLOIDY IN THE DEGENERATIVE PHASE OF SERIAL CULTIVATION OF HUMAN CELL STRAINS. Proc Natl Acad Sci U S A. 1963 Aug;50:390–395. doi: 10.1073/pnas.50.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood S. W., Rush D., Ellsworth J. L., Schimke R. T. Defining cellular senescence in IMR-90 cells: a flow cytometric analysis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9086–9090. doi: 10.1073/pnas.85.23.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. The cellular and subcellular bases of immunosenescence. Adv Immunol. 1989;46:221–261. doi: 10.1016/s0065-2776(08)60655-0. [DOI] [PubMed] [Google Scholar]
- Ugazio A. G., Maccario R., Notarangelo L. D., Burgio G. R. Immunology of Down syndrome: a review. Am J Med Genet Suppl. 1990;7:204–212. doi: 10.1002/ajmg.1320370742. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]