Abstract
The association of some diseases with specific alleles of certain genetic markers has been difficult to explain. Several explanations have been proposed for the phenomenon of association, e.g. the existence of multiple, interacting genes (epistasis) or a disease locus in linkage disequilibrium with the marker locus. One might suppose that when marker data from families with associated diseases are analyzed for linkage, the existence of the association would assure that linkage will be found, and found at a tight recombination fraction. In fact, however, linkage analyses of some diseases associated with HLA, as well as diseases associated with alleles at other loci located throughout the genome, show significant evidence against linkage, and others show loose linkage, to the puzzlement of many researchers. In part, the puzzlement arises because linkage analysis is ideal for looking for loci that are necessary, even if not sufficient, for disease expression but may be much less useful for finding loci that are neither necessary nor sufficient for disease expression (so-called susceptibility loci). This work explores what happens when one looks for linkage to susceptibility loci. A susceptibility locus in this case means that the allele increases risk but is neither necessary nor sufficient for disease expression. It might be either an allele at the marker locus itself that is increasing susceptibility or an allele at a locus in linkage disequilibrium with the marker. This work uses computer simulation to examine how linkage analyses behave when confronted with data from such a model.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




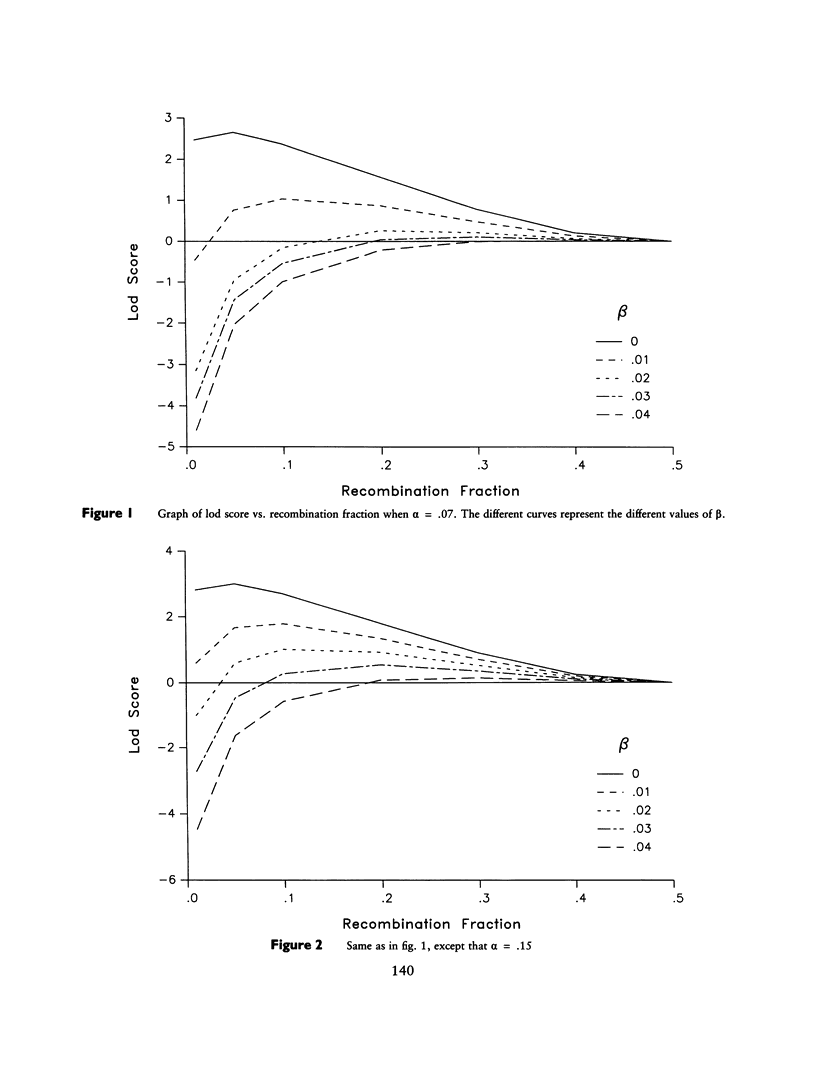
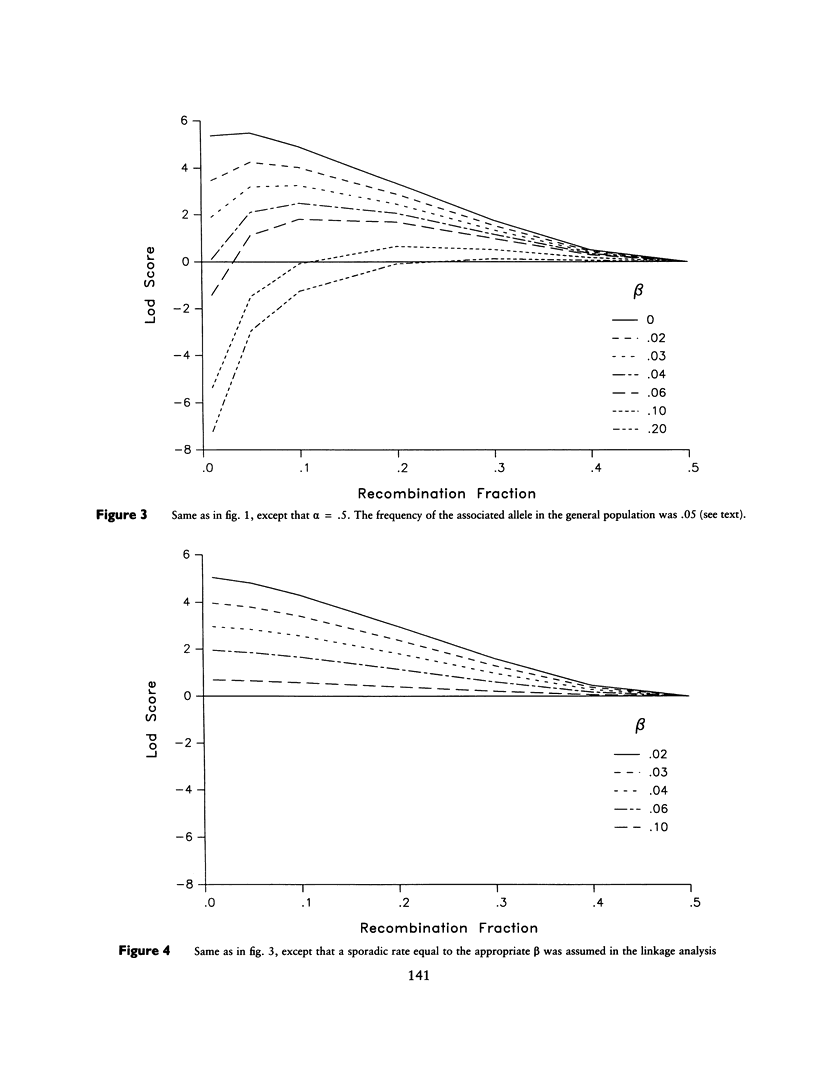


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allannic H., Fauchet R., Lorcy Y., Heim J., Gueguen M., Leguerrier A. M., Genetet B. HLA and Graves' disease: an association with HLA-DRw3. J Clin Endocrinol Metab. 1980 Oct;51(4):863–867. doi: 10.1210/jcem-51-4-863. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Spielman R. S. The insulin gene and susceptibility to IDDM. Genet Epidemiol. 1989;6(1):65–69. doi: 10.1002/gepi.1370060113. [DOI] [PubMed] [Google Scholar]
- Durner M., Greenberg D. A. Effect of heterogeneity and assumed mode of inheritance on lod scores. Am J Med Genet. 1992 Feb 1;42(3):271–275. doi: 10.1002/ajmg.1320420302. [DOI] [PubMed] [Google Scholar]
- Farid N. R., Sampson L., Noel E. P., Barnard J. M., Mandeville R., Larsen B., Marshall W. H., Carter N. D. A study of human leukocyte D locus related antigens in Graves' disease. J Clin Invest. 1979 Jan;63(1):108–113. doi: 10.1172/JCI109263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. L. Non-HLA region genes in insulin dependent diabetes mellitus. Baillieres Clin Endocrinol Metab. 1991 Sep;5(3):413–438. doi: 10.1016/s0950-351x(05)80139-9. [DOI] [PubMed] [Google Scholar]
- Go R. C., Alarcón G. S., Acton R. T., Koopman W. J., Vittor V. J., Barger B. O. Analyses of HLA linkage in white families with multiple cases of seropositive rheumatoid arthritis. Arthritis Rheum. 1987 Oct;30(10):1115–1123. doi: 10.1002/art.1780301005. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A., Hodge S. E. Linkage analysis under "random" and "genetic" reduced penetrance. Genet Epidemiol. 1989;6(1):259–264. doi: 10.1002/gepi.1370060145. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A., Rotter J. I. Two locus models for gluten sensitive enteropathy: population genetic considerations. Am J Med Genet. 1981;8(2):205–214. doi: 10.1002/ajmg.1320080211. [DOI] [PubMed] [Google Scholar]
- Greenberg D. A. Simulation studies of segregation analysis: application to two-locus models. Am J Hum Genet. 1984 Jan;36(1):167–176. [PMC free article] [PubMed] [Google Scholar]
- Hodge S. E., Spence M. A., Cederbaum S. D. Hypertrophic myocardiopathy. N Engl J Med. 1979 Aug 23;301(8):442–443. doi: 10.1056/NEJM197908233010829. [DOI] [PubMed] [Google Scholar]
- Hodge S. E., Spence M. A. Some epistatic two-locus models of disease. II. The confounding of linkage and association. Am J Hum Genet. 1981 May;33(3):396–406. [PMC free article] [PubMed] [Google Scholar]
- Langdon N., Welsh K. I., van Dam M., Vaughan R. W., Parkes D. Genetic markers in narcolepsy. Lancet. 1984 Nov 24;2(8413):1178–1180. doi: 10.1016/s0140-6736(84)92742-9. [DOI] [PubMed] [Google Scholar]
- Morton N. E., Green A., Dunsworth T., Svejgaard A., Barbosa J., Rich S. S., Iselius L., Platz P., Ryder L. P. Heterozygous expression of insulin-dependent diabetes mellitus (IDDM) determinants in the HLA system. Am J Hum Genet. 1983 Mar;35(2):201–213. [PMC free article] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- Rigby A. S., Silman A. J., Voelm L., Gregory J. C., Ollier W. E., Khan M. A., Nepom G. T., Thomson G. Investigating the HLA component in rheumatoid arthritis: an additive (dominant) mode of inheritance is rejected, a recessive mode is preferred. Genet Epidemiol. 1991;8(3):153–175. doi: 10.1002/gepi.1370080303. [DOI] [PubMed] [Google Scholar]
- Roman S. H., Greenberg D., Rubinstein P., Wallenstein S., Davies T. F. Genetics of autoimmune thyroid disease: lack of evidence for linkage to HLA within families. J Clin Endocrinol Metab. 1992 Mar;74(3):496–503. doi: 10.1210/jcem.74.3.1740483. [DOI] [PubMed] [Google Scholar]
- Thomson G. A two locus model for juvenile diabetes. Ann Hum Genet. 1980 May;43(4):383–398. doi: 10.1111/j.1469-1809.1980.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Tiwari J. L., Hodge S. E., Terasaki P. I., Spence M. A. HLA and the inheritance of multiple sclerosis: linkage analysis of 72 pedigrees. Am J Hum Genet. 1980 Jan;32(1):103–111. [PMC free article] [PubMed] [Google Scholar]
- Wagener D. K., Sacks J. M., LaPorte R. E., Macgregor J. M. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes. 1982 Feb;31(2):136–144. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]


