Abstract
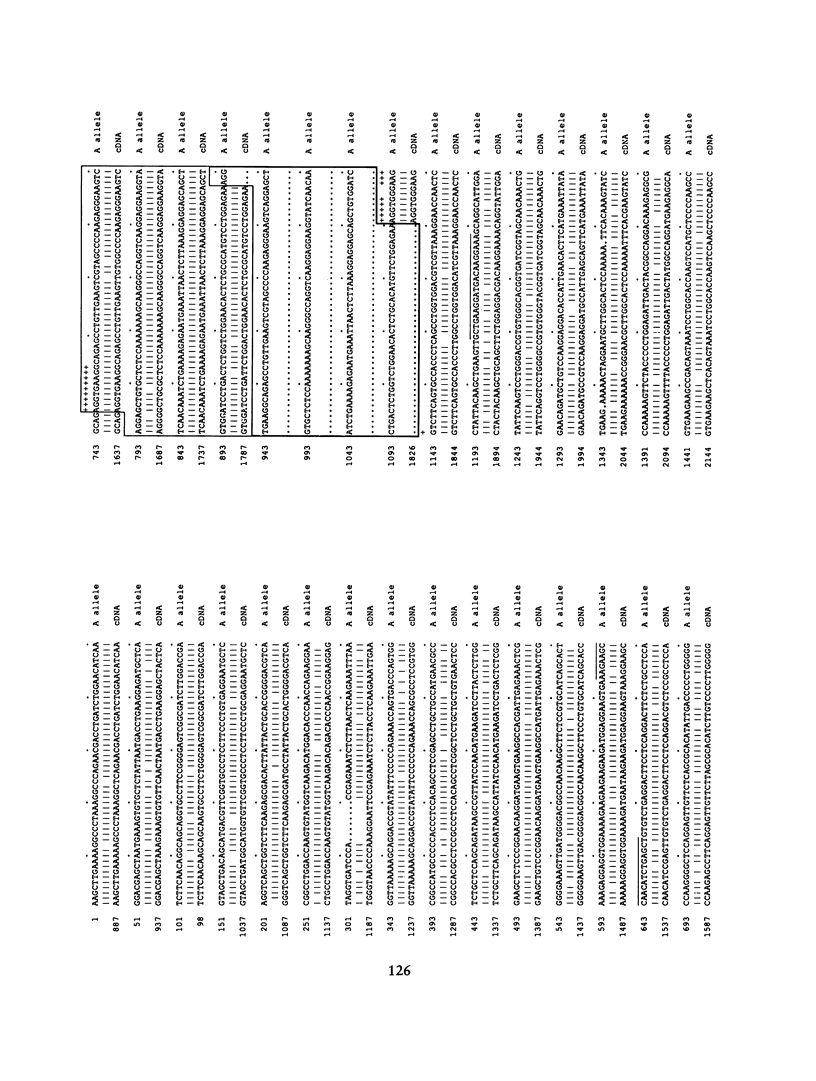
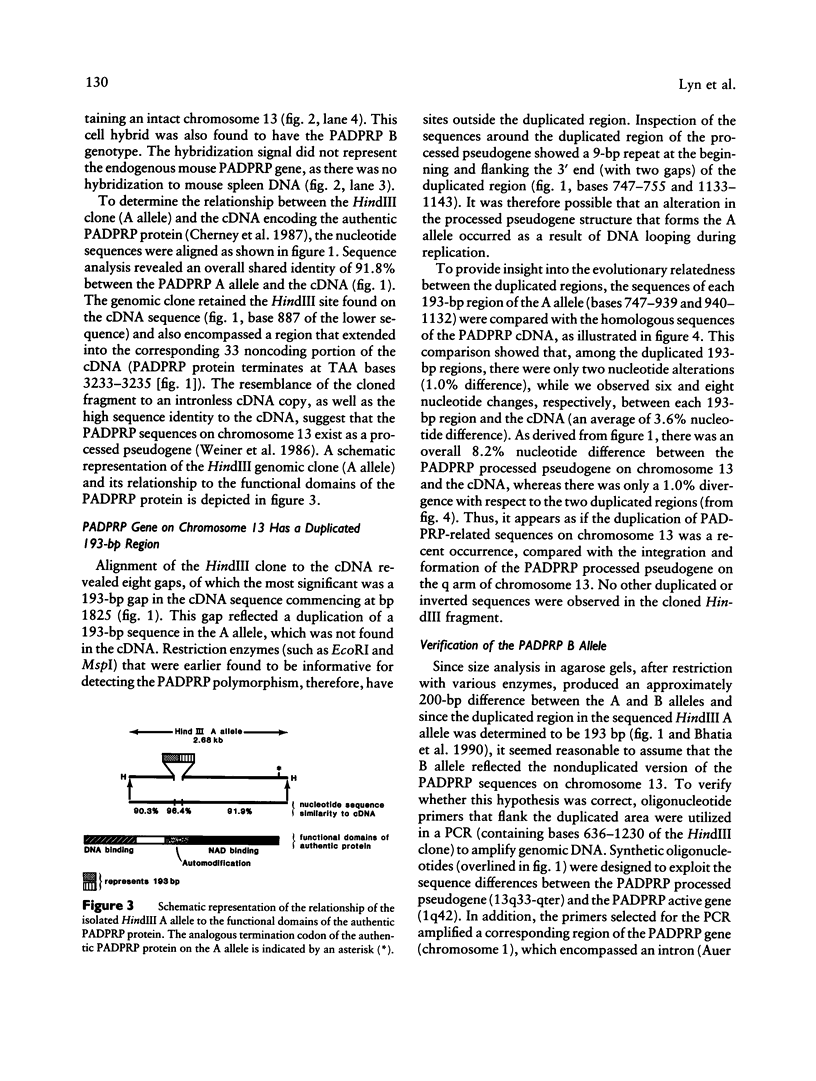
The poly(ADP-ribose) polymerase (PADPRP) gene (13q33-qter) depicts a two-allele (A/B) polymorphism. In the noncancer population, the frequency of the B allele is higher among blacks than among whites. Since the incidence of multiple myeloma and prostate and lung cancer is higher in the U.S. black population, we have analyzed the B-allele frequency in germ-line DNA to determine whether the PADPRP gene correlates with a polymorphic susceptibility to these diseases. For multiple myeloma and prostate cancer, an increased frequency of the B allele appeared to be striking only in black patients. In contrast, the distribution of the B allele in germ-line DNA did not differ among white patients with these diseases, when compared with the control group. An elevated B-allele frequency was also found in germ-line DNA in blacks with colon cancer. These observations suggest that the PADPRP polymorphism may provide a valid marker for a predisposition to these cancers in black individuals. To determine the genomic structure of the polymorphic PADPRP sequences, a 2.68-kb HindIII clone was isolated and sequenced from a chromosome 13-enriched library. Sequence analysis of this clone (A allele) revealed a close sequence similarity (91.8%) to PADPRP cDNA (1q42) and an absence of introns, suggesting that the gene on 13q exists as a processed pseudogene. A 193-bp conserved duplicated region within the A allele was identified as the source of the polymorphism. The nucleotide differences between the PADPRP gene on chromosome 13 and related PADPRP genes were exploited to develop oligonucleotides that can detect the difference between the A/B genotypes in a PCR. This PCR assay offers the opportunity for analyzing additional black cancer patients, to determine how the PADPRP processed pseudogene or an unidentified gene that cosegregates with the PADPRP gene might be involved with the development of malignancy.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer B., Nagl U., Herzog H., Schneider R., Schweiger M. Human nuclear NAD+ ADP-ribosyltransferase(polymerizing): organization of the gene. DNA. 1989 Oct;8(8):575–580. doi: 10.1089/dna.1989.8.575. [DOI] [PubMed] [Google Scholar]
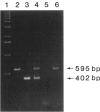
- Bhatia K. G., Cherney B. W., Huppi K., Magrath I. T., Cossman J., Sausville E., Barriga F., Johnson B., Gause B., Bonney G. A deletion linked to a poly(ADP-ribose) polymerase gene on chromosome 13q33-qter occurs frequently in the normal black population as well as in multiple tumor DNA. Cancer Res. 1990 Sep 1;50(17):5406–5413. [PubMed] [Google Scholar]
- Chakraborty R., Kamboh M. I., Nwankwo M., Ferrell R. E. Caucasian genes in American blacks: new data. Am J Hum Genet. 1992 Jan;50(1):145–155. [PMC free article] [PubMed] [Google Scholar]
- Cherney B. W., McBride O. W., Chen D. F., Alkhatib H., Bhatia K., Hensley P., Smulson M. E. cDNA sequence, protein structure, and chromosomal location of the human gene for poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8370–8374. doi: 10.1073/pnas.84.23.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Smith B. A., Cooke H. J., Niemann S., Schmidtke J. An estimate of unique DNA sequence heterozygosity in the human genome. Hum Genet. 1985;69(3):201–205. doi: 10.1007/BF00293024. [DOI] [PubMed] [Google Scholar]
- Cowell J. K., Mitchell C. D. A somatic cell hybrid mapping panel for regional assignment of human chromosome 13 DNA sequences. Cytogenet Cell Genet. 1989;52(1-2):1–6. doi: 10.1159/000132827. [DOI] [PubMed] [Google Scholar]
- Lalande M., Dryja T. P., Schreck R. R., Shipley J., Flint A., Latt S. A. Isolation of human chromosome 13-specific DNA sequences cloned from flow sorted chromosomes and potentially linked to the retinoblastoma locus. Cancer Genet Cytogenet. 1984 Dec;13(4):283–295. doi: 10.1016/0165-4608(84)90073-6. [DOI] [PubMed] [Google Scholar]
- Maeda N., Bliska J. B., Smithies O. Recombination and balanced chromosome polymorphism suggested by DNA sequences 5' to the human delta-globin gene. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5012–5016. doi: 10.1073/pnas.80.16.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M. M., Koop B. F., Slightom J. L., Goodman M., Tennant M. R. Molecular systematics of higher primates: genealogical relations and classification. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7627–7631. doi: 10.1073/pnas.85.20.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottern L. M., Gart J. J., Nam J. M., Dunston G., Wilson J., Greenberg R., Schoenberg J., Swanson G. M., Liff J., Schwartz A. G. HLA and multiple myeloma among black and white men: evidence of a genetic association. Cancer Epidemiol Biomarkers Prev. 1992 Mar-Apr;1(3):177–182. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]