Abstract
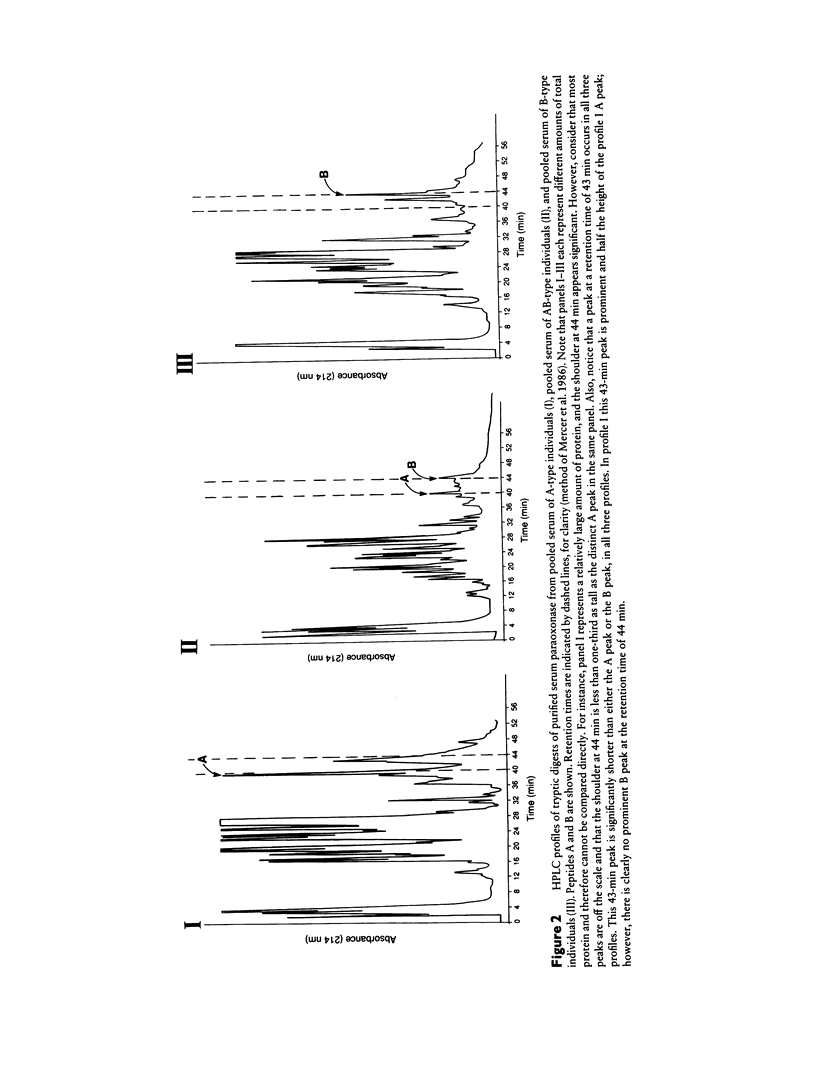
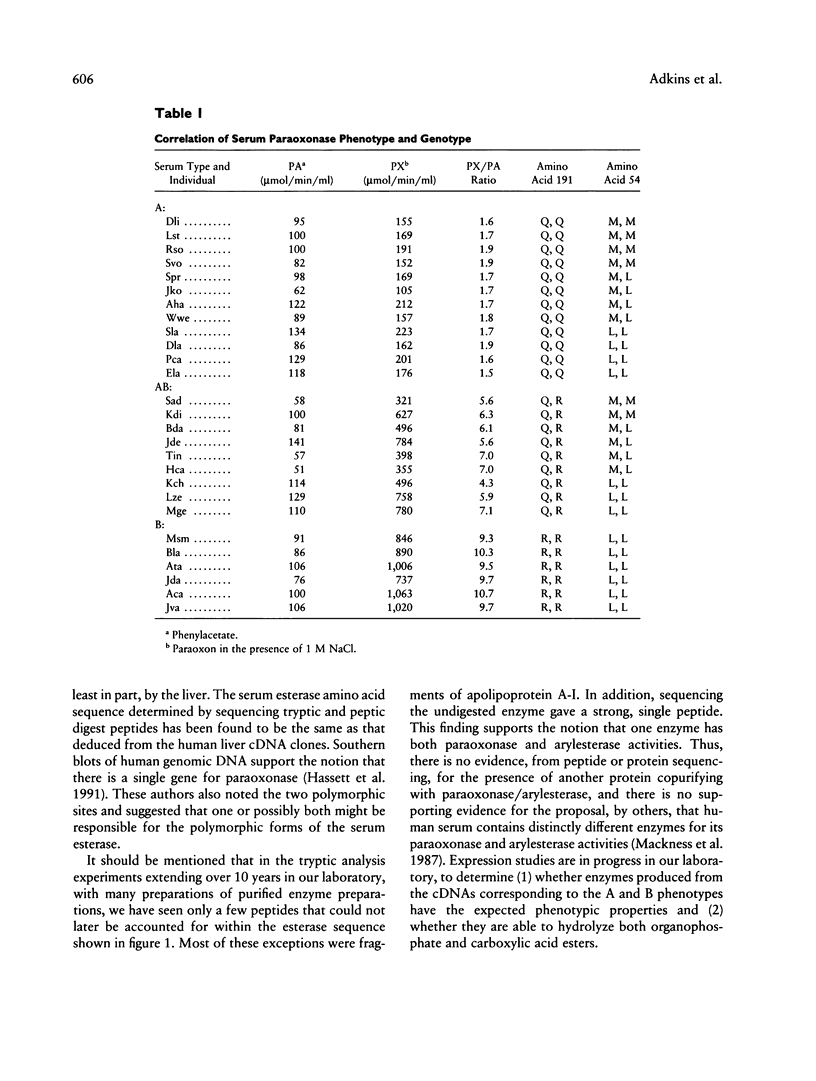
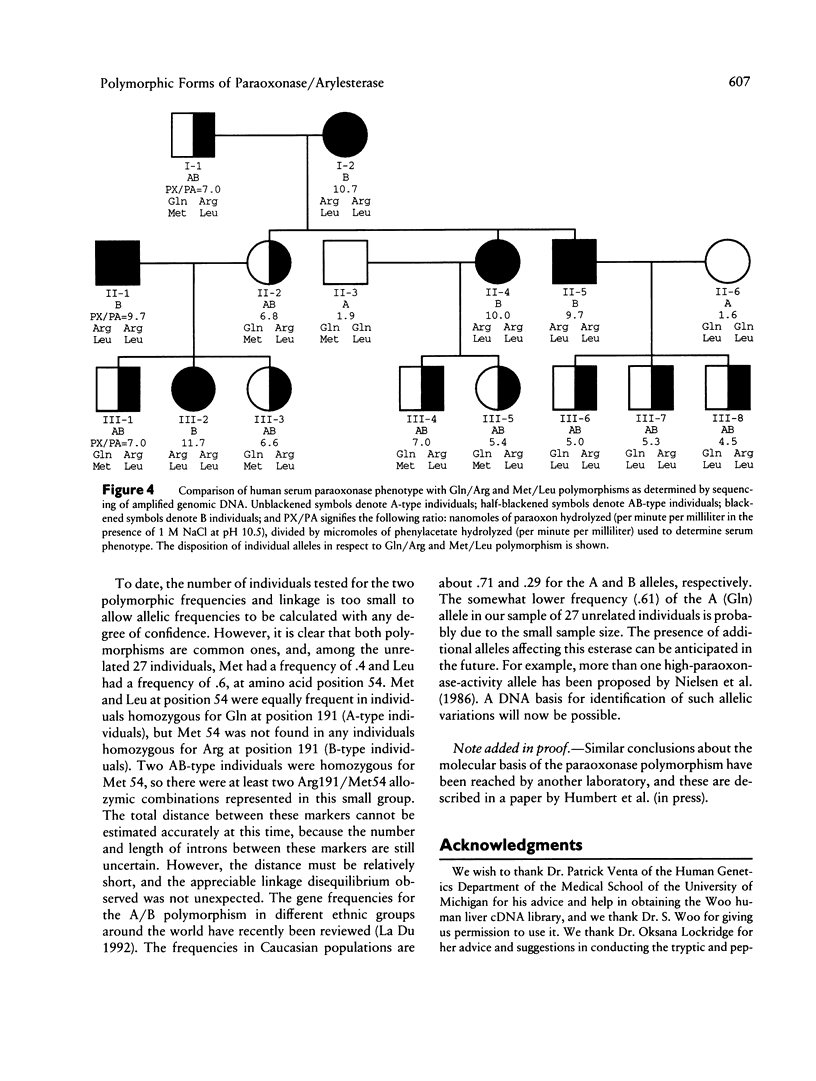
The paraoxonase/arylesterase gene is located close to the cystic fibrosis gene on chromosome 7. Human serum contains two paraoxonase/arylesterase allozymes, A and B, which differ in their substrate specificities and kinetic properties. Purified A, AB, and B esterases were digested with trypsin, and the resultant peptides were compared by high-performance liquid chromatography. The elution profiles were very similar for all three samples, except for (1) one peptide (i.e., peptide A) seen only in the A and AB profiles and (2) another peptide (i.e., peptide B) seen only in the B and AB profiles. Sequencing revealed that peptide A had glutamine at amino acid position 191, whereas peptide B was generated by cleavage on the carboxy side of position 191, presumably because there was a basic (trypsin-specific) amino acid at that position. Working independently, our laboratory and one other laboratory have sequenced the coding region for paraoxonase from human liver cDNA libraries and have identified two polymorphic sites: Arg/Gln at position 191 and Leu/Met at position 54. Using PCR amplification and direct sequencing of nucleotides in both polymorphic regions with genomic DNA, we have estimated the allelic frequencies and have determined their concordance with the serum paraoxonase allozyme phenotypes in 27 unrelated adults and in 16 members of a three-generation pedigree. Among unrelated individuals, the Met/Leu polymorphism at position 54 did not correlate with the serum esterase phenotype. In contrast, the particular amino acid at position 191 correlated perfectly with serum phenotypes: A-type individuals had Gln at position 191, and B-type individuals had Arg at position 191; AB-type serum was found only with the heterozygous (Arg/Gln) combination. Pedigree analysis showed both polymorphisms to be inherited in the expected Mendelian manner and confirmed that only the 191 polymorphism showed concordance with the serum paraoxonase/arylesterase phenotypes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S. D., Coon M. J. Simple, rapid, and highly efficient separation of amino acid phenylthiohydantoins by reversed-phase high-performance liquid chromatography. Anal Biochem. 1982 Apr;121(2):281–285. doi: 10.1016/0003-2697(82)90480-8. [DOI] [PubMed] [Google Scholar]
- Eckerson H. W., Romson J., Wyte C., La Du B. N. The human serum paraoxonase polymorphism: identification of phenotypes by their response to salts. Am J Hum Genet. 1983 Mar;35(2):214–227. [PMC free article] [PubMed] [Google Scholar]
- Eckerson H. W., Wyte C. M., La Du B. N. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983 Nov;35(6):1126–1138. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Richter R. J., Chapline C., Crabb J. W. Purification of rabbit and human serum paraoxonase. Biochemistry. 1991 Oct 22;30(42):10133–10140. doi: 10.1021/bi00106a009. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Richter R. J., Seidel S. L., Motulsky A. G. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am J Hum Genet. 1988 Sep;43(3):230–238. [PMC free article] [PubMed] [Google Scholar]
- Gan K. N., Smolen A., Eckerson H. W., La Du B. N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991 Jan-Feb;19(1):100–106. [PubMed] [Google Scholar]
- Hamilton B. A., Palazzolo M. J., Meyerowitz E. M. Rapid isolation of long cDNA clones from existing libraries. Nucleic Acids Res. 1991 Apr 25;19(8):1951–1952. doi: 10.1093/nar/19.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett C., Richter R. J., Humbert R., Chapline C., Crabb J. W., Omiecinski C. J., Furlong C. E. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry. 1991 Oct 22;30(42):10141–10149. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- Kwok S. C., Ledley F. D., DiLella A. G., Robson K. J., Woo S. L. Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry. 1985 Jan 29;24(3):556–561. doi: 10.1021/bi00324a002. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987 Jan 15;262(2):549–557. [PubMed] [Google Scholar]
- Mackness M. I., Thompson H. M., Hardy A. R., Walker C. H. Distinction between 'A'-esterases and arylesterases. Implications for esterase classification. Biochem J. 1987 Jul 1;245(1):293–296. doi: 10.1042/bj2450293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M. C., Nogueira C. P., Bartels C. F., Lightstone H., Hajra A., Van der Spek A. F., Lockridge O., La Du B. N. Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase. Proc Natl Acad Sci U S A. 1989 Feb;86(3):953–957. doi: 10.1073/pnas.86.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., McAdam W., Chambers G. W., Walker I. D. The W and L allelic forms of phenylalanine hydroxylase in the rat differ by a threonine to isoleucine substitution. Biochem J. 1986 Jun 15;236(3):679–683. doi: 10.1042/bj2360679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllenbach R., Lagoda P. J., Welter C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989 Dec;5(12):391–391. [PubMed] [Google Scholar]
- Playfer J. R., Eze L. C., Bullen M. F., Evans D. A. Genetic polymorphism and interethnic variability of plasma paroxonase activity. J Med Genet. 1976 Oct;13(5):337–342. doi: 10.1136/jmg.13.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Smolen A., Eckerson H. W., Gan K. N., Hailat N., La Du B. N. Characteristics of the genetically determined allozymic forms of human serum paraoxonase/arylesterase. Drug Metab Dispos. 1991 Jan-Feb;19(1):107–112. [PubMed] [Google Scholar]
- Tsui L. C., Buchwald M., Barker D., Braman J. C., Knowlton R., Schumm J. W., Eiberg H., Mohr J., Kennedy D., Plavsic N. Cystic fibrosis locus defined by a genetically linked polymorphic DNA marker. Science. 1985 Nov 29;230(4729):1054–1057. doi: 10.1126/science.2997931. [DOI] [PubMed] [Google Scholar]



