Abstract
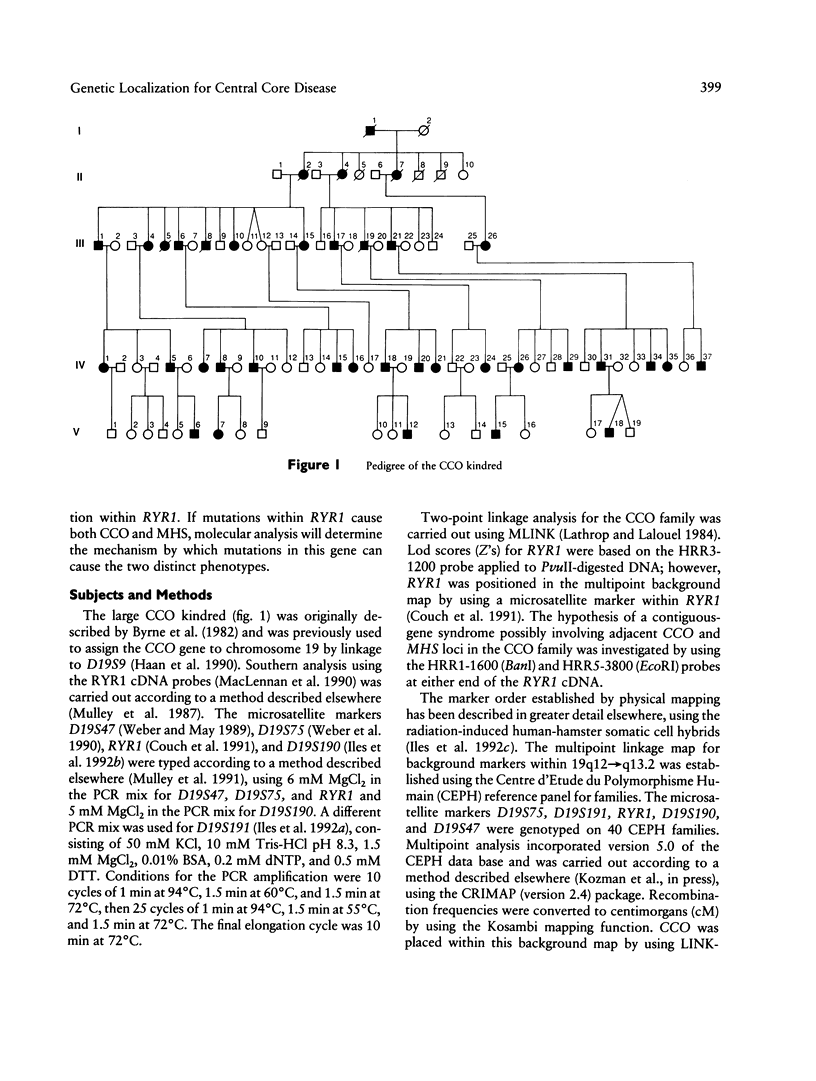
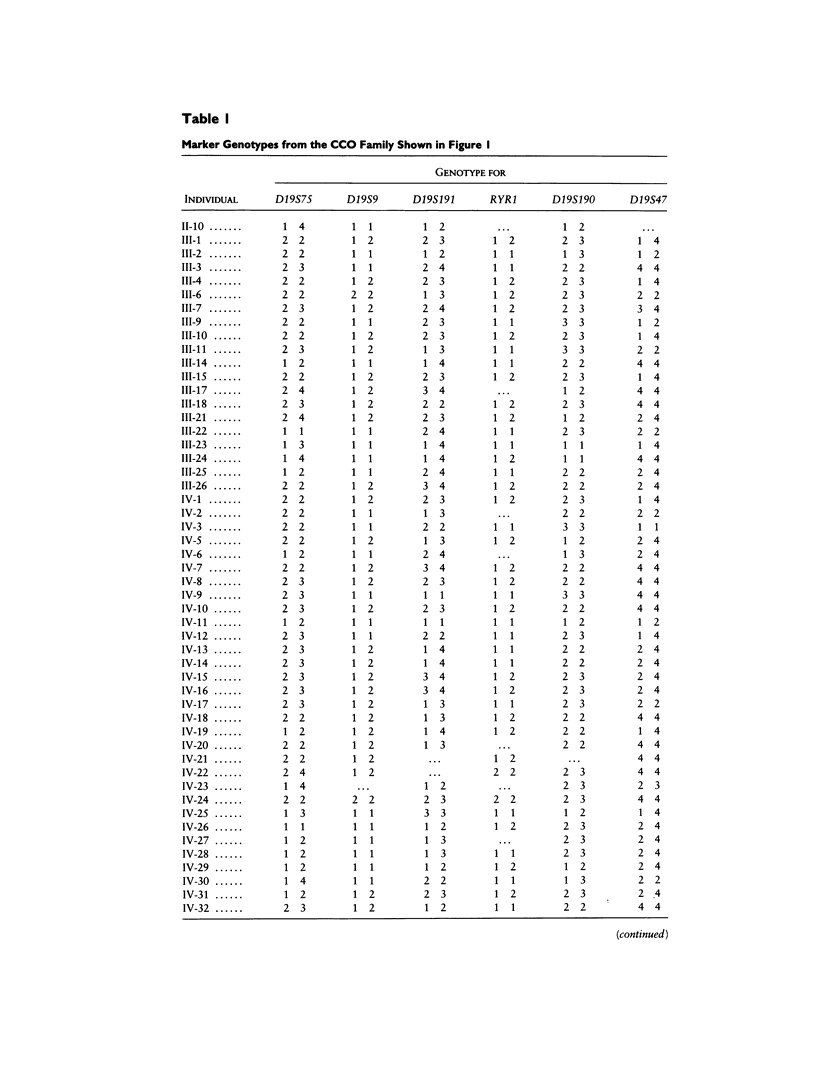
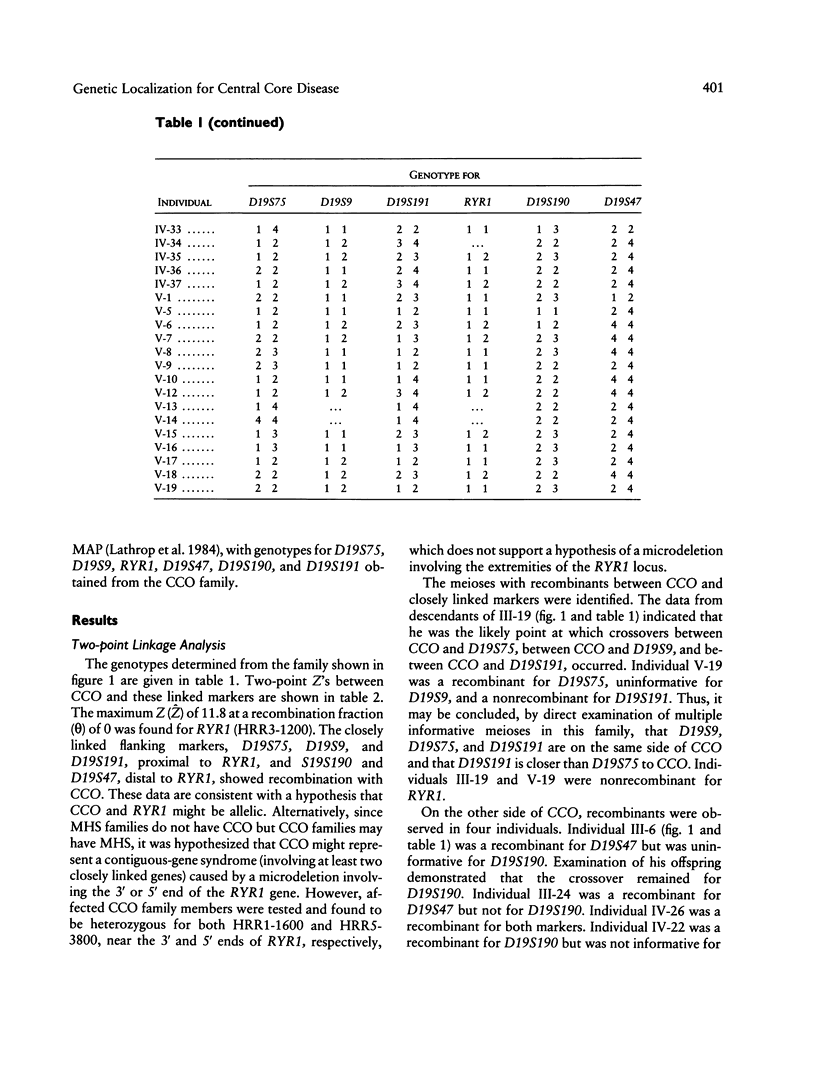
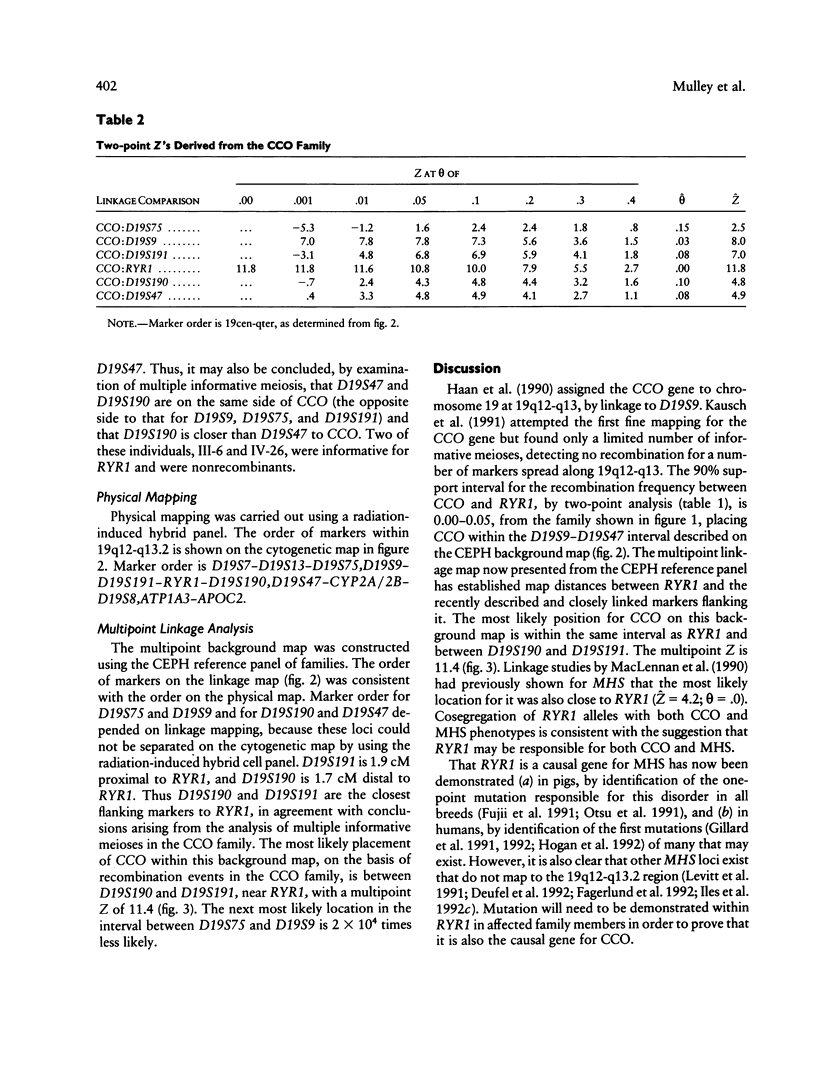
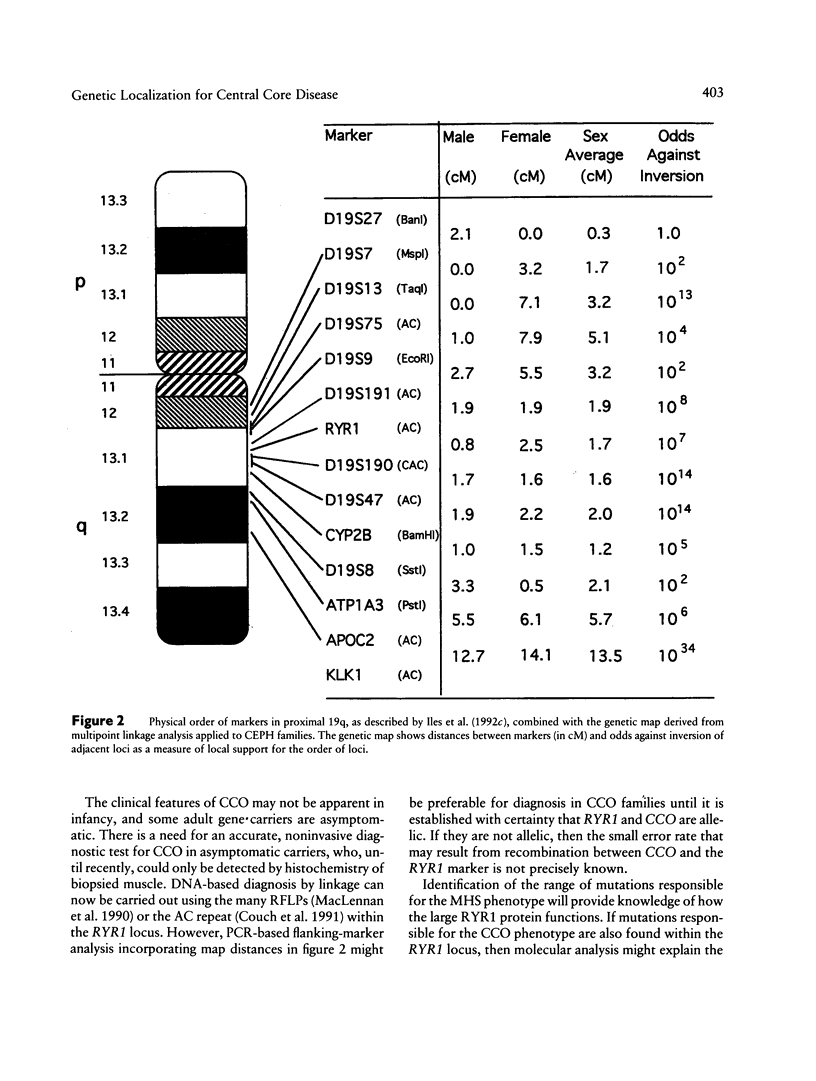
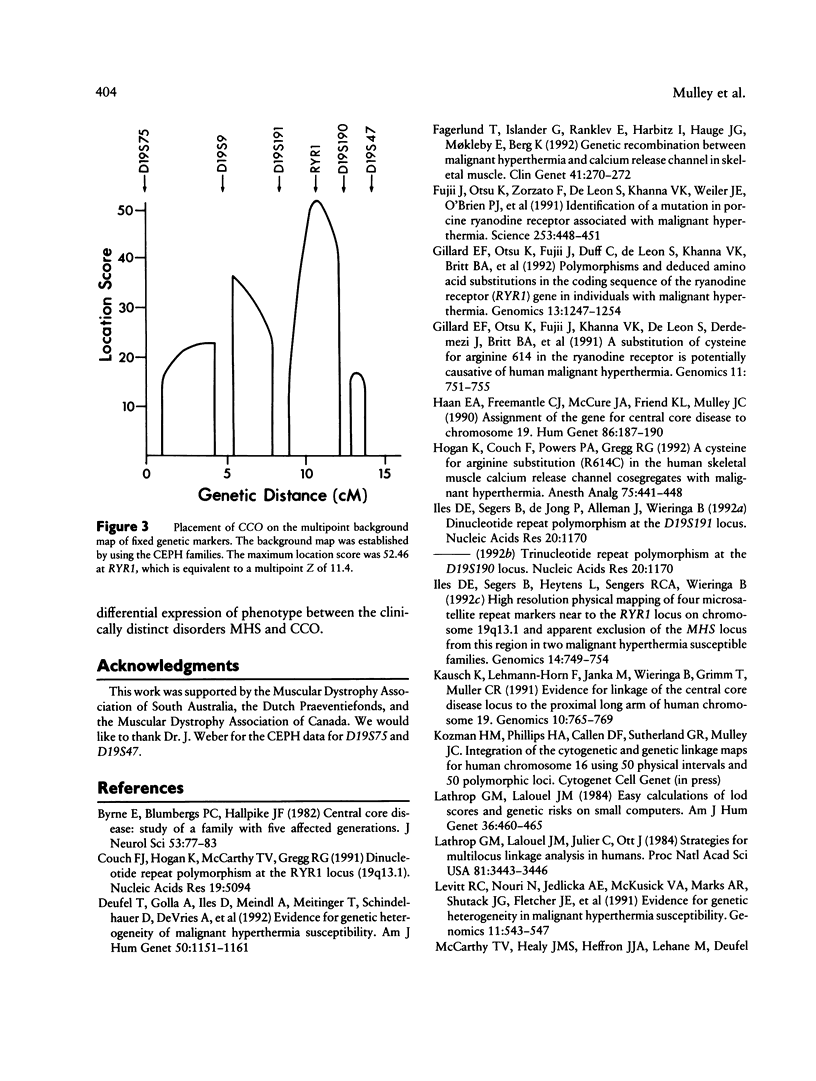
Central core disease (CCO) is an autosomal dominant myopathy clinically distinct from malignant hyperthermia (MHS). In a large kindred in which the gene for CCO is segregating, two-point linkage analysis gave a maximum lod score, between the central core disease locus (CCO) and the ryanodine receptor locus (RYR1), of 11.8, with no recombination. Mutation within RYR1 is responsible for MHS, and RYR1 is also a candidate locus for CCO. A combination of physical mapping using a radiation-induced human-hamster hybrid panel and of multipoint linkage analysis using the Centre d'Etude du Polymorphisme Humain families established the marker order and sex-average map distances (in centimorgans) on the background map as D19S75–(5.2)–D19S9–(3.4)–D19S191–(2.2)–RYR1–(1.7)–D19S190–(1.6)-D19S47–(2.0)–CYP2B. Recombination was observed between CCO and the markers flanking RYR1. These linkage data are consistent with the hypothesis that CCO and RYR1 are allelic. The most likely position for CCO is near RYR1, with a multipoint lod score of 11.4, in 19q13.1 between D19S191 and D19S190, within the same interval as MHS (RYR1).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne E., Blumbergs P. C., Hallpike J. F. Central core disease. Study of a family with five affected generations. J Neurol Sci. 1982 Jan;53(1):77–83. doi: 10.1016/0022-510x(82)90081-8. [DOI] [PubMed] [Google Scholar]
- Couch F. J., Hogan K., McCarthy T. V., Gregg R. G. Dinucleotide repeat polymorphism at the RYR1 locus (19q13.1). Nucleic Acids Res. 1991 Sep 25;19(18):5094–5094. doi: 10.1093/nar/19.18.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deufel T., Golla A., Iles D., Meindl A., Meitinger T., Schindelhauer D., DeVries A., Pongratz D., MacLennan D. H., Johnson K. J. Evidence for genetic heterogeneity of malignant hyperthermia susceptibility. Am J Hum Genet. 1992 Jun;50(6):1151–1161. [PMC free article] [PubMed] [Google Scholar]
- Fagerlund T., Islander G., Ranklev E., Harbitz I., Hauge J. G., Møkleby E., Berg K. Genetic recombination between malignant hyperthermia and calcium release channel in skeletal muscle. Clin Genet. 1992 May;41(5):270–272. [PubMed] [Google Scholar]
- Fujii J., Otsu K., Zorzato F., de Leon S., Khanna V. K., Weiler J. E., O'Brien P. J., MacLennan D. H. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991 Jul 26;253(5018):448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- Gillard E. F., Otsu K., Fujii J., Duff C., de Leon S., Khanna V. K., Britt B. A., Worton R. G., MacLennan D. H. Polymorphisms and deduced amino acid substitutions in the coding sequence of the ryanodine receptor (RYR1) gene in individuals with malignant hyperthermia. Genomics. 1992 Aug;13(4):1247–1254. doi: 10.1016/0888-7543(92)90042-q. [DOI] [PubMed] [Google Scholar]
- Gillard E. F., Otsu K., Fujii J., Khanna V. K., de Leon S., Derdemezi J., Britt B. A., Duff C. L., Worton R. G., MacLennan D. H. A substitution of cysteine for arginine 614 in the ryanodine receptor is potentially causative of human malignant hyperthermia. Genomics. 1991 Nov;11(3):751–755. doi: 10.1016/0888-7543(91)90084-r. [DOI] [PubMed] [Google Scholar]
- Haan E. A., Freemantle C. J., McCure J. A., Friend K. L., Mulley J. C. Assignment of the gene for central core disease to chromosome 19. Hum Genet. 1990 Dec;86(2):187–190. doi: 10.1007/BF00197703. [DOI] [PubMed] [Google Scholar]
- Hogan K., Couch F., Powers P. A., Gregg R. G. A cysteine-for-arginine substitution (R614C) in the human skeletal muscle calcium release channel cosegregates with malignant hyperthermia. Anesth Analg. 1992 Sep;75(3):441–448. doi: 10.1213/00000539-199209000-00022. [DOI] [PubMed] [Google Scholar]
- Iles D. E., Segers B., Heytens L., Sengers R. C., Wieringa B. High-resolution physical mapping of four microsatellite repeat markers near the RYR1 locus on chromosome 19q13.1 and apparent exclusion of the MHS locus from this region in two malignant hyperthermia susceptible families. Genomics. 1992 Nov;14(3):749–754. doi: 10.1016/s0888-7543(05)80179-x. [DOI] [PubMed] [Google Scholar]
- Iles D. E., Segers B., de Jong P., Alleman J., Wieringa B. Dinucleotide repeat polymorphism at the D19S191 locus. Nucleic Acids Res. 1992 Mar 11;20(5):1170–1170. [PMC free article] [PubMed] [Google Scholar]
- Kausch K., Lehmann-Horn F., Janka M., Wieringa B., Grimm T., Müller C. R. Evidence for linkage of the central core disease locus to the proximal long arm of human chromosome 19. Genomics. 1991 Jul;10(3):765–769. doi: 10.1016/0888-7543(91)90461-m. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt R. C., Nouri N., Jedlicka A. E., McKusick V. A., Marks A. R., Shutack J. G., Fletcher J. E., Rosenberg H., Meyers D. A. Evidence for genetic heterogeneity in malignant hyperthermia susceptibility. Genomics. 1991 Nov;11(3):543–547. doi: 10.1016/0888-7543(91)90061-i. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Duff C., Zorzato F., Fujii J., Phillips M., Korneluk R. G., Frodis W., Britt B. A., Worton R. G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990 Feb 8;343(6258):559–561. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- McCarthy T. V., Healy J. M., Heffron J. J., Lehane M., Deufel T., Lehmann-Horn F., Farrall M., Johnson K. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature. 1990 Feb 8;343(6258):562–564. doi: 10.1038/343562a0. [DOI] [PubMed] [Google Scholar]
- Mulley J. C., Gedeon A. K., Thorn K. A., Bates L. J., Sutherland G. R. Linkage and genetic counselling for the fragile X using DNA probes 52A, F9, DX13, and ST14. Am J Med Genet. 1987 Jun;27(2):435–448. doi: 10.1002/ajmg.1320270222. [DOI] [PubMed] [Google Scholar]
- Mulley J. C., Gedeon A. K., White S. J., Haan E. A., Richards R. I. Predictive diagnosis of myotonic dystrophy with flanking microsatellite markers. J Med Genet. 1991 Jul;28(7):448–452. doi: 10.1136/jmg.28.7.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu K., Khanna V. K., Archibald A. L., MacLennan D. H. Cosegregation of porcine malignant hyperthermia and a probable causal mutation in the skeletal muscle ryanodine receptor gene in backcross families. Genomics. 1991 Nov;11(3):744–750. doi: 10.1016/0888-7543(91)90083-q. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Kappel C., May P. E., Kwitek A. E. Dinucleotide repeat polymorphism at the D19S75 locus. Nucleic Acids Res. 1990 Aug 11;18(15):4639–4639. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]



