Abstract
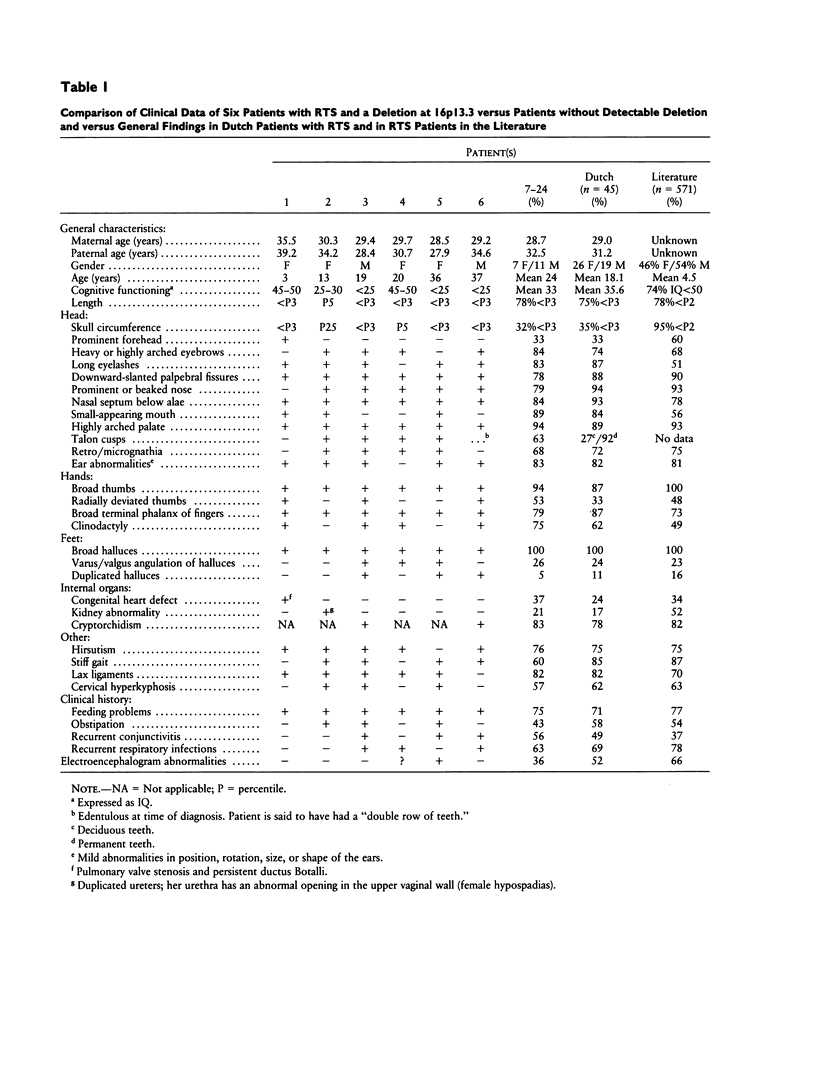
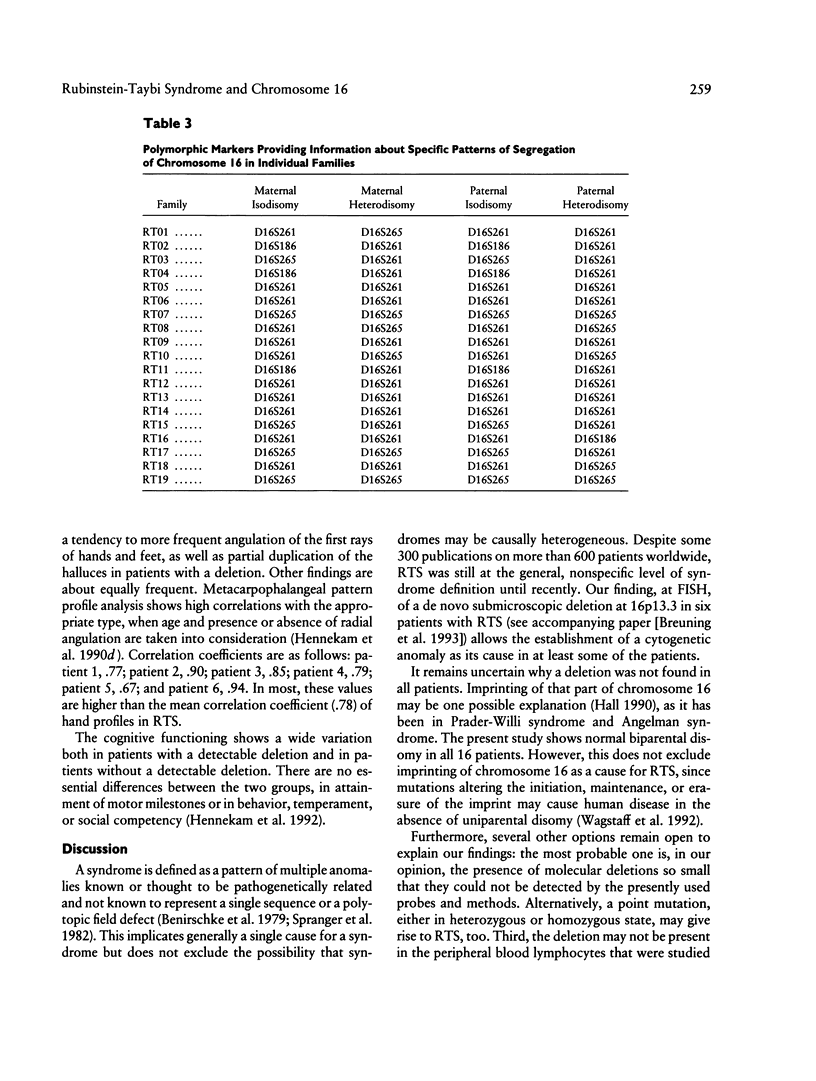
In the accompanying paper, a chromosomal localization of the Rubinstein-Taybi syndrome by cytogenetic investigations with fluorescence in situ hybridization techniques at chromosome 16p13.3 is described. We investigated 19 of these patients and their parents (a) to ascertain the parental origin of the chromosome with the deletion in families where such a deletion was detected, (b) to disclose whether uniparental disomy plays a role in etiology, and (c) to compare clinical features in patients with a deletion to those in individuals in whom deletions were not detectable. Molecular studies showed a copy of chromosome 16 from each parent in all 19 patients. Uniparental disomy was also excluded for five other chromosome arms known to be imprinted in mice. None of the probes used for determining the origin of the deleted chromosome proved to be informative. The clinical features were essentially the same in patients with and without visible deletion, with a possible exception for the incidence of microcephaly, angulation of thumbs and halluces, and partial duplication of the halluces. A small deletion at 16p13.3 may be found in some patients with Rubinstein-Taybi syndrome. Cytogenetically undetectable deletions, point mutations, mosaicism, heterogeneity, or phenocopy by a nongenetic cause are the most probable explanations for the absence of cytogenetic or molecular abnormalities in other patients with Rubinstein-Taybi syndrome.
Full text
PDF





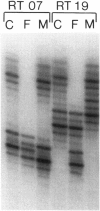
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballabio A. Contiguous deletion syndromes. Curr Opin Genet Dev. 1991 Jun;1(1):25–29. doi: 10.1016/0959-437x(91)80036-l. [DOI] [PubMed] [Google Scholar]
- Bazacliu E., Tonceanu S., Carp G., Ghişoiu V., Roşca G., Roşca S. Sindrom Rubinstein-Taybi cu modificări de cariotip şi pneumopatie recidivantă. Ftiziologia. 1973 Nov-Dec;22(6):645–650. [PubMed] [Google Scholar]
- Benirschke K., Lowry R. B., Opitz J. M., Schwarzacher H. G., Spranger J. W. Developmental terms -- some proposals: first report of an international working group. Am J Med Genet. 1979;3(3):297–302. doi: 10.1002/ajmg.1320030309. [DOI] [PubMed] [Google Scholar]
- Bilir B. M., Bilir N., Wilson G. N. Intracranial angioblastic meningioma and an aged appearance in a woman with Rubinstein-Taybi syndrome. Am J Med Genet Suppl. 1990;6:69–72. doi: 10.1002/ajmg.1320370612. [DOI] [PubMed] [Google Scholar]
- Breuning M. H., Dauwerse H. G., Fugazza G., Saris J. J., Spruit L., Wijnen H., Tommerup N., van der Hagen C. B., Imaizumi K., Kuroki Y. Rubinstein-Taybi syndrome caused by submicroscopic deletions within 16p13.3. Am J Hum Genet. 1993 Feb;52(2):249–254. [PMC free article] [PubMed] [Google Scholar]
- Davison B. C., Ellis H. L., Kuzemko J. A., Roberts D. F. Mental retardation with facial abnormalities, broad thumbs and toes and unusual dermatoglyphics. Dev Med Child Neurol. 1967 Oct;9(5):588–593. doi: 10.1111/j.1469-8749.1967.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Graff-Radford S. B. Melkersson-Rosenthal syndrome. A review of the literature and case report. S Afr Med J. 1981 Jul 11;60(2):71–74. [PubMed] [Google Scholar]
- Hall J. G. Genomic imprinting: review and relevance to human diseases. Am J Hum Genet. 1990 May;46(5):857–873. [PMC free article] [PubMed] [Google Scholar]
- Hennekam R. C., Baselier A. C., Beyaert E., Bos A., Blok J. B., Jansma H. B., Thorbecke-Nilsen V. V., Veerman H. Psychological and speech studies in Rubinstein-Taybi syndrome. Am J Ment Retard. 1992 May;96(6):645–660. [PubMed] [Google Scholar]
- Hennekam R. C., Lommen E. J., Strengers J. L., Van Spijker H. G., Jansen-Kokx T. M. Rubinstein-Taybi syndrome in a mother and son. Eur J Pediatr. 1989 Feb;148(5):439–441. doi: 10.1007/BF00595907. [DOI] [PubMed] [Google Scholar]
- Hennekam R. C., Stevens C. A., Van de Kamp J. J. Etiology and recurrence risk in Rubinstein-Taybi syndrome. Am J Med Genet Suppl. 1990;6:56–64. doi: 10.1002/ajmg.1320370610. [DOI] [PubMed] [Google Scholar]
- Hennekam R. C., Van Den Boogaard M. J., Dijkstra P. F., Van de Kamp J. J. Metacarpophalangeal pattern profile analysis in Rubinstein-Taybi syndrome. Am J Med Genet Suppl. 1990;6:48–50. doi: 10.1002/ajmg.1320370608. [DOI] [PubMed] [Google Scholar]
- Hennekam R. C., Van Den Boogaard M. J., Sibbles B. J., Van Spijker H. G. Rubinstein-Taybi syndrome in The Netherlands. Am J Med Genet Suppl. 1990;6:17–29. doi: 10.1002/ajmg.1320370604. [DOI] [PubMed] [Google Scholar]
- Hennekam R. C., Van den Boogaard M. J., Van Doorne J. M. A cephalometric study in Rubinstein-Taybi syndrome. J Craniofac Genet Dev Biol. 1991 Jan-Mar;11(1):33–40. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINSTEIN J. H., TAYBI H. Broad thumbs and toes and facial abnormalities. A possible mental retardation syndrome. Am J Dis Child. 1963 Jun;105:588–608. doi: 10.1001/archpedi.1963.02080040590010. [DOI] [PubMed] [Google Scholar]
- Reeders S. T., Breuning M. H., Davies K. E., Nicholls R. D., Jarman A. P., Higgs D. R., Pearson P. L., Weatherall D. J. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985 Oct 10;317(6037):542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- Rubinstein J. H. Broad thumb-hallux (Rubinstein-Taybi) syndrome 1957-1988. Am J Med Genet Suppl. 1990;6:3–16. doi: 10.1002/ajmg.1320370603. [DOI] [PubMed] [Google Scholar]
- Schmickel R. D. Contiguous gene syndromes: a component of recognizable syndromes. J Pediatr. 1986 Aug;109(2):231–241. doi: 10.1016/s0022-3476(86)80377-8. [DOI] [PubMed] [Google Scholar]
- Searle A. G., Peters J., Lyon M. F., Hall J. G., Evans E. P., Edwards J. H., Buckle V. J. Chromosome maps of man and mouse. IV. Ann Hum Genet. 1989 May;53(Pt 2):89–140. doi: 10.1111/j.1469-1809.1989.tb01777.x. [DOI] [PubMed] [Google Scholar]
- Spranger J., Benirschke K., Hall J. G., Lenz W., Lowry R. B., Opitz J. M., Pinsky L., Schwarzacher H. G., Smith D. W. Errors of morphogenesis: concepts and terms. Recommendations of an international working group. J Pediatr. 1982 Jan;100(1):160–165. doi: 10.1016/s0022-3476(82)80261-8. [DOI] [PubMed] [Google Scholar]
- Stevens C. A., Hennekam R. C., Blackburn B. L. Growth in the Rubinstein-Taybi syndrome. Am J Med Genet Suppl. 1990;6:51–55. doi: 10.1002/ajmg.1320370609. [DOI] [PubMed] [Google Scholar]
- Wagstaff J., Knoll J. H., Glatt K. A., Shugart Y. Y., Sommer A., Lalande M. Maternal but not paternal transmission of 15q11-13-linked nondeletion Angelman syndrome leads to phenotypic expression. Nat Genet. 1992 Jul;1(4):291–294. doi: 10.1038/ng0792-291. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]