Abstract
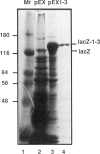
The first three exons of the human muscle dystrophin gene were expressed as a beta-galactosidase fusion protein. This protein was then used to prepare two monoclonal antibodies (mAbs) which react with native dystrophin on frozen muscle sections and with denatured dystrophin on western blots but which do not cross-react with the dystrophin-related protein, utrophin. Both mAbs recognized dystrophin in muscular dystrophy (MD) patients with deletions of exon 3, and further mapping with 11 overlapping synthetic peptides showed that they both recognize an epitope encoded by the muscle-specific exon 1. Neither mAb recognizes the brain dystrophin isoform, confirming the prediction from mRNA data that this has a different N-terminus. One Becker MD patient with a frameshift deletion of exons 3-7 is shown to produce dystrophin which reacts with the N-terminal mAbs, as well as with mAbs which bind on the C-terminal side of the deletion. The data suggest that transcription begins at the normal muscle dystrophin promoter and that the normal reading frame is restored after the deletion. A number of mechanisms have been proposed for restoration of the reading frame after deletion of exons 3-7, but those which predict dystrophin with an abnormal N-terminus do not appear to be major mechanisms in this patient.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Beggs A. H., Honda H., Ito S., Ishiura S., Tsukahara T., Ishiguro T., Eguchi C., Orimo S., Arikawa E. Preservation of the C-terminus of dystrophin molecule in the skeletal muscle from Becker muscular dystrophy. J Neurol Sci. 1991 Feb;101(2):148–156. doi: 10.1016/0022-510x(91)90039-a. [DOI] [PubMed] [Google Scholar]
- Barnea E., Zuk D., Simantov R., Nudel U., Yaffe D. Specificity of expression of the muscle and brain dystrophin gene promoters in muscle and brain cells. Neuron. 1990 Dec;5(6):881–888. doi: 10.1016/0896-6273(90)90348-j. [DOI] [PubMed] [Google Scholar]
- Beggs A. H., Hoffman E. P., Kunkel L. M. Additional dystrophin fragment in Becker muscular dystrophy may result from proteolytic cleavage at deletion junctions. Am J Med Genet. 1992 Oct 1;44(3):378–381. doi: 10.1002/ajmg.1320440322. [DOI] [PubMed] [Google Scholar]
- Beggs A. H., Hoffman E. P., Snyder J. R., Arahata K., Specht L., Shapiro F., Angelini C., Sugita H., Kunkel L. M. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am J Hum Genet. 1991 Jul;49(1):54–67. [PMC free article] [PubMed] [Google Scholar]
- Bies R. D., Phelps S. F., Cortez M. D., Roberts R., Caskey C. T., Chamberlain J. S. Human and murine dystrophin mRNA transcripts are differentially expressed during skeletal muscle, heart, and brain development. Nucleic Acids Res. 1992 Apr 11;20(7):1725–1731. doi: 10.1093/nar/20.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. J., Love D. R., Tinsley J., Morris G. E., Turley H., Gatter K., Dickson G., Edwards Y. H., Davies K. E. Characterization of a 4.8kb transcript from the Duchenne muscular dystrophy locus expressed in Schwannoma cells. Hum Mol Genet. 1992 May;1(2):103–109. doi: 10.1093/hmg/1.2.103. [DOI] [PubMed] [Google Scholar]
- Bulman D. E., Gangopadhyay S. B., Bebchuck K. G., Worton R. G., Ray P. N. Point mutation in the human dystrophin gene: identification through western blot analysis. Genomics. 1991 Jun;10(2):457–460. doi: 10.1016/0888-7543(91)90332-9. [DOI] [PubMed] [Google Scholar]
- Chelly J., Gilgenkrantz H., Lambert M., Hamard G., Chafey P., Récan D., Katz P., de la Chapelle A., Koenig M., Ginjaar I. B. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990 Dec 21;63(6):1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay S. B., Sherratt T. G., Heckmatt J. Z., Dubowitz V., Miller G., Shokeir M., Ray P. N., Strong P. N., Worton R. G. Dystrophin in frameshift deletion patients with Becker muscular dystrophy. Am J Hum Genet. 1992 Sep;51(3):562–570. [PMC free article] [PubMed] [Google Scholar]
- Górecki D. C., Monaco A. P., Derry J. M., Walker A. P., Barnard E. A., Barnard P. J. Expression of four alternative dystrophin transcripts in brain regions regulated by different promoters. Hum Mol Genet. 1992 Oct;1(7):505–510. doi: 10.1093/hmg/1.7.505. [DOI] [PubMed] [Google Scholar]
- Helliwell T. R., Ellis J. M., Mountford R. C., Appleton R. E., Morris G. E. A truncated dystrophin lacking the C-terminal domains is localized at the muscle membrane. Am J Hum Genet. 1992 Mar;50(3):508–514. [PMC free article] [PubMed] [Google Scholar]
- Hugnot J. P., Gilgenkrantz H., Vincent N., Chafey P., Morris G. E., Monaco A. P., Berwald-Netter Y., Koulakoff A., Kaplan J. C., Kahn A. Distal transcript of the dystrophin gene initiated from an alternative first exon and encoding a 75-kDa protein widely distributed in nonmuscle tissues. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7506–7510. doi: 10.1073/pnas.89.16.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lederfein D., Levy Z., Augier N., Mornet D., Morris G., Fuchs O., Yaffe D., Nudel U. A 71-kilodalton protein is a major product of the Duchenne muscular dystrophy gene in brain and other nonmuscle tissues. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5346–5350. doi: 10.1073/pnas.89.12.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. R., Morris G. E., Ellis J. M., Fairbrother U., Marsden R. F., Bloomfield J. F., Edwards Y. H., Slater C. P., Parry D. J., Davies K. E. Tissue distribution of the dystrophin-related gene product and expression in the mdx and dy mouse. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3243–3247. doi: 10.1073/pnas.88.8.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen thi Man, Cartwright A. J., Morris G. E., Love D. R., Bloomfield J. F., Davies K. E. Monoclonal antibodies against defined regions of the muscular dystrophy protein, dystrophin. FEBS Lett. 1990 Mar 26;262(2):237–240. doi: 10.1016/0014-5793(90)80199-s. [DOI] [PubMed] [Google Scholar]
- Nguyen T. M., Ellis J. M., Ginjaar I. B., van Paassen M. M., van Ommen G. J., Moorman A. F., Cartwright A. J., Morris G. E. Monoclonal antibody evidence for structural similarities between the central rod regions of actinin and dystrophin. FEBS Lett. 1990 Oct 15;272(1-2):109–112. doi: 10.1016/0014-5793(90)80460-z. [DOI] [PubMed] [Google Scholar]
- Nguyen T. M., Ellis J. M., Love D. R., Davies K. E., Gatter K. C., Dickson G., Morris G. E. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol. 1991 Dec;115(6):1695–1700. doi: 10.1083/jcb.115.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. M., Ginjaar I. B., van Ommen G. J., Morris G. E. Monoclonal antibodies for dystrophin analysis. Epitope mapping and improved binding to SDS-treated muscle sections. Biochem J. 1992 Dec 1;288(Pt 2):663–668. doi: 10.1042/bj2880663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L. V., Bushby K. M., Johnson M. A., den Dunnen J. T., Ginjaar I. B., van Ommen G. J. Predicted and observed sizes of dystrophin in some patients with gene deletions that disrupt the open reading frame. J Med Genet. 1992 Dec;29(12):892–896. doi: 10.1136/jmg.29.12.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Zuk D., Einat P., Zeelon E., Levy Z., Neuman S., Yaffe D. Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature. 1989 Jan 5;337(6202):76–78. doi: 10.1038/337076a0. [DOI] [PubMed] [Google Scholar]
- Read A. P., Mountford R. C., Forrest S. M., Kenwrick S. J., Davies K. E., Harris R. Patterns of exon deletions in Duchenne and Becker muscular dystrophy. Hum Genet. 1988 Oct;80(2):152–156. doi: 10.1007/BF00702859. [DOI] [PubMed] [Google Scholar]
- Roberts R. G., Coffey A. J., Bobrow M., Bentley D. R. Determination of the exon structure of the distal portion of the dystrophin gene by vectorette PCR. Genomics. 1992 Aug;13(4):942–950. doi: 10.1016/0888-7543(92)90005-d. [DOI] [PubMed] [Google Scholar]
- Récan D., Chafey P., Leturcq F., Hugnot J. P., Vincent N., Tomé F., Collin H., Simon D., Czernichow P., Nicholson L. V. Are cysteine-rich and COOH-terminal domains of dystrophin critical for sarcolemmal localization? J Clin Invest. 1992 Feb;89(2):712–716. doi: 10.1172/JCI115640. [DOI] [PMC free article] [PubMed] [Google Scholar]