Abstract
In patients with myotonic dystrophy (DM), the severity of clinical signs is correlated with the length of a (CTG)n trinucleotide repeat sequence. This sequence tends to expand in subsequent generations. In order to examine the kinetics of this process and, in particular, the influence of the mutant-allele size and the sex of the transmitting parent, we have studied (CTG)n repeat lengths in the offspring of 38 healthy carriers with small mutations (less than 100 CTG trinucleotides, mean length [CTG]67). In these studies, we found a weakly positive correlation between the size of the mutation in the carrier parents and that in their offspring. Furthermore, we observed that, in the offspring of male transmitters, repeat lengths exceeding 100 CTG trinucleotides were much more frequent than in the offspring of carrier females (48 [92%] of 52 vs. 7 [44%] of 16, P = .0002). Similarly, in genealogical studies performed in 38 Dutch DM kindreds, an excess of nonmanifesting male transmitters was noted, which was most conspicuous in the generation immediately preceding that with phenotypic expression of DM. Thus, two separate lines of evidence suggest that the sex of the transmitting parent is an important factor that determines DM allele size in the offspring. On the basis of our data, we estimate that when both parents are asymptomatic, the odds are approximately 2:1 that the father carries the DM mutation. Because expansion of the CTG repeat is more rapid with male transmission, negative selection during spermatogenesis may be required to explain the exclusive maternal inheritance of severe congenital onset DM.
Full text
PDF

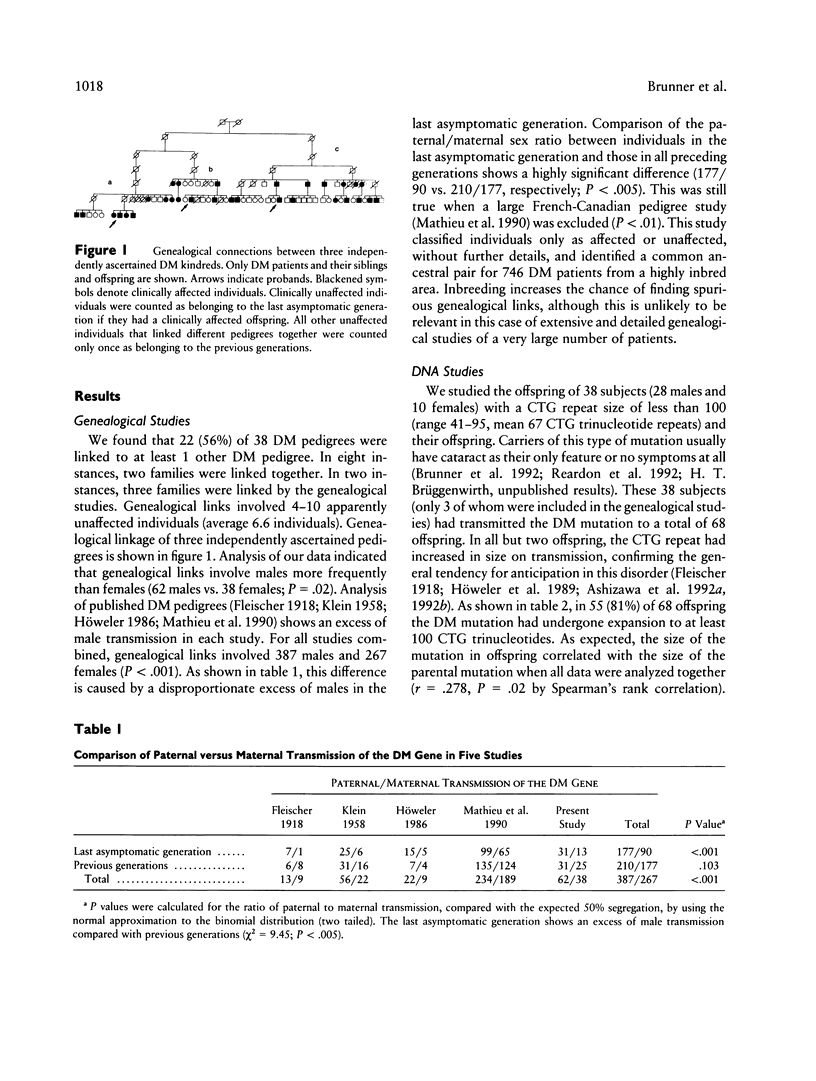
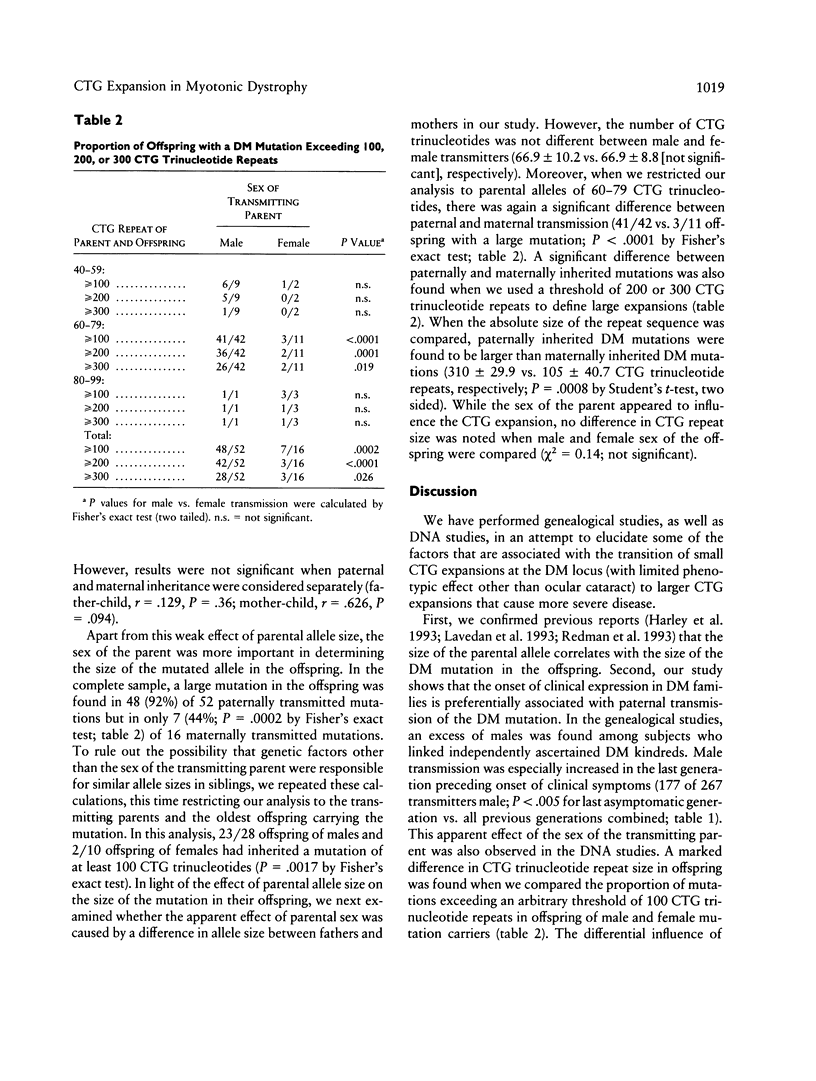




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich D., Lerer I., Pashut-Lavon I., Shmueli E., Raas-Rothschild A., Frydman M. Negative expansion of the myotonic dystrophy unstable sequence. Am J Hum Genet. 1993 Jun;52(6):1175–1181. [PMC free article] [PubMed] [Google Scholar]
- Ashizawa T., Dubel J. R., Dunne P. W., Dunne C. J., Fu Y. H., Pizzuti A., Caskey C. T., Boerwinkle E., Perryman M. B., Epstein H. F. Anticipation in myotonic dystrophy. II. Complex relationships between clinical findings and structure of the GCT repeat. Neurology. 1992 Oct;42(10):1877–1883. doi: 10.1212/wnl.42.10.1877. [DOI] [PubMed] [Google Scholar]
- Ashizawa T., Dunne C. J., Dubel J. R., Perryman M. B., Epstein H. F., Boerwinkle E., Hejtmancik J. F. Anticipation in myotonic dystrophy. I. Statistical verification based on clinical and haplotype findings. Neurology. 1992 Oct;42(10):1871–1877. doi: 10.1212/wnl.42.10.1871. [DOI] [PubMed] [Google Scholar]
- Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992 Feb 6;355(6360):548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Jansen G., Nillesen W., Nelen M. R., de Die C. E., Höweler C. J., van Oost B. A., Wieringa B., Ropers H. H., Smeets H. J. Brief report: reverse mutation in myotonic dystrophy. N Engl J Med. 1993 Feb 18;328(7):476–480. doi: 10.1056/NEJM199302183280705. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Nillesen W., van Oost B. A., Jansen G., Wieringa B., Ropers H. H., Smeets H. J. Presymptomatic diagnosis of myotonic dystrophy. J Med Genet. 1992 Nov;29(11):780–784. doi: 10.1136/jmg.29.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner H. G., Smeets H. J., Nillesen W., van Oost B. A., van den Biezenbos J. B., Joosten E. M., Pinckers A. J., Hamel B. C., Theeuwes A. G., Wieringa B. Myotonic dystrophy. Predictive value of normal results on clinical examination. Brain. 1991 Oct;114(Pt 5):2303–2311. doi: 10.1093/brain/114.5.2303. [DOI] [PubMed] [Google Scholar]
- Buxton J., Shelbourne P., Davies J., Jones C., Van Tongeren T., Aslanidis C., de Jong P., Jansen G., Anvret M., Riley B. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature. 1992 Feb 6;355(6360):547–548. doi: 10.1038/355547a0. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Pizzuti A., Fenwick R. G., Jr, King J., Rajnarayan S., Dunne P. W., Dubel J., Nasser G. A., Ashizawa T., de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992 Mar 6;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Griggs R. C., Wood D. S. Criteria for establishing the validity of genetic recombination in myotonic dystrophy. Neurology. 1989 Mar;39(3):420–421. doi: 10.1212/wnl.39.3.420. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Rundle S. A., Crow S., Reardon W., Buckler A. J., Harper P. S., Housman D. E., Shaw D. J. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992 Feb 6;355(6360):545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Rundle S. A., MacMillan J. C., Myring J., Brook J. D., Crow S., Reardon W., Fenton I., Shaw D. J., Harper P. S. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet. 1993 Jun;52(6):1164–1174. [PMC free article] [PubMed] [Google Scholar]
- Harley H. G., Rundle S. A., Reardon W., Myring J., Crow S., Brook J. D., Harper P. S., Shaw D. J. Unstable DNA sequence in myotonic dystrophy. Lancet. 1992 May 9;339(8802):1125–1128. doi: 10.1016/0140-6736(92)90729-m. [DOI] [PubMed] [Google Scholar]
- Heitz D., Devys D., Imbert G., Kretz C., Mandel J. L. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant of the transition to full mutation. J Med Genet. 1992 Nov;29(11):794–801. doi: 10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A. G., Jacob P., O'Hoy K., MacDonald I., Mettler G., Tsilfidis C., Korneluk R. G. Decrease in the size of the myotonic dystrophy CTG repeat during transmission from parent to child: implications for genetic counselling and genetic anticipation. Am J Med Genet. 1993 Feb 1;45(3):401–407. doi: 10.1002/ajmg.1320450330. [DOI] [PubMed] [Google Scholar]
- Hunter A., Tsilfidis C., Mettler G., Jacob P., Mahadevan M., Surh L., Korneluk R. The correlation of age of onset with CTG trinucleotide repeat amplification in myotonic dystrophy. J Med Genet. 1992 Nov;29(11):774–779. doi: 10.1136/jmg.29.11.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höweler C. J., Busch H. F., Geraedts J. P., Niermeijer M. F., Staal A. Anticipation in myotonic dystrophy: fact or fiction? Brain. 1989 Jun;112(Pt 3):779–797. doi: 10.1093/brain/112.3.779. [DOI] [PubMed] [Google Scholar]
- Jansen G., Bartolomei M., Kalscheuer V., Merkx G., Wormskamp N., Mariman E., Smeets D., Ropers H. H., Wieringa B. No imprinting involved in the expression of DM-kinase mRNAs in mouse and human tissues. Hum Mol Genet. 1993 Aug;2(8):1221–1227. doi: 10.1093/hmg/2.8.1221. [DOI] [PubMed] [Google Scholar]
- Jansen G., Mahadevan M., Amemiya C., Wormskamp N., Segers B., Hendriks W., O'Hoy K., Baird S., Sabourin L., Lennon G. Characterization of the myotonic dystrophy region predicts multiple protein isoform-encoding mRNAs. Nat Genet. 1992 Jul;1(4):261–266. doi: 10.1038/ng0792-261. [DOI] [PubMed] [Google Scholar]
- Lavedan C., Hofmann-Radvanyi H., Shelbourne P., Rabes J. P., Duros C., Savoy D., Dehaupas I., Luce S., Johnson K., Junien C. Myotonic dystrophy: size- and sex-dependent dynamics of CTG meiotic instability, and somatic mosaicism. Am J Hum Genet. 1993 May;52(5):875–883. [PMC free article] [PubMed] [Google Scholar]
- Leske M. C., Sperduto R. D. The epidemiology of senile cataracts: a review. Am J Epidemiol. 1983 Aug;118(2):152–165. doi: 10.1093/oxfordjournals.aje.a113625. [DOI] [PubMed] [Google Scholar]
- Lyttle T. W. Cheaters sometimes prosper: distortion of mendelian segregation by meiotic drive. Trends Genet. 1993 Jun;9(6):205–210. doi: 10.1016/0168-9525(93)90120-7. [DOI] [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Mathieu J., De Braekeleer M., Prévost C. Genealogical reconstruction of myotonic dystrophy in the Saguenay-Lac-Saint-Jean area (Quebec, Canada). Neurology. 1990 May;40(5):839–842. doi: 10.1212/wnl.40.5.839. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hoy K. L., Tsilfidis C., Mahadevan M. S., Neville C. E., Barceló J., Hunter A. G., Korneluk R. G. Reduction in size of the myotonic dystrophy trinucleotide repeat mutation during transmission. Science. 1993 Feb 5;259(5096):809–812. doi: 10.1126/science.8094260. [DOI] [PubMed] [Google Scholar]
- Reardon W., Harley H. G., Brook J. D., Rundle S. A., Crow S., Harper P. S., Shaw D. J. Minimal expression of myotonic dystrophy: a clinical and molecular analysis. J Med Genet. 1992 Nov;29(11):770–773. doi: 10.1136/jmg.29.11.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman J. B., Fenwick R. G., Jr, Fu Y. H., Pizzuti A., Caskey C. T. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA. 1993 Apr 21;269(15):1960–1965. [PubMed] [Google Scholar]
- Reik W. Genomic imprinting and genetic disorders in man. Trends Genet. 1989 Oct;5(10):331–336. doi: 10.1016/0168-9525(89)90138-8. [DOI] [PubMed] [Google Scholar]
- Shelbourne P., Winqvist R., Kunert E., Davies J., Leisti J., Thiele H., Bachmann H., Buxton J., Williamson B., Johnson K. Unstable DNA may be responsible for the incomplete penetrance of the myotonic dystrophy phenotype. Hum Mol Genet. 1992 Oct;1(7):467–473. doi: 10.1093/hmg/1.7.467. [DOI] [PubMed] [Google Scholar]
- Tsilfidis C., MacKenzie A. E., Mettler G., Barceló J., Korneluk R. G. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat Genet. 1992 Jun;1(3):192–195. doi: 10.1038/ng0692-192. [DOI] [PubMed] [Google Scholar]
- Yu S., Mulley J., Loesch D., Turner G., Donnelly A., Gedeon A., Hillen D., Kremer E., Lynch M., Pritchard M. Fragile-X syndrome: unique genetics of the heritable unstable element. Am J Hum Genet. 1992 May;50(5):968–980. [PMC free article] [PubMed] [Google Scholar]
- van Straaten A. Het verloop van het aantal consanguïene huwelijken in Nederland (1906-1982). Ned Tijdschr Geneeskd. 1986 Nov 1;130(44):1984–1986. [PubMed] [Google Scholar]


