Abstract
Neisserial porins are strong immune adjuvants and B cell activators. The effect of neisserial porin PorB on activation-induced cell death was investigated, as a potential additional mechanism of the porin's immunopotentiating ability. Neisserial porins interact with target cells to localize intracellularly in the mitochondrial compartment without negatively affecting cellular survival. Pretreatment with Neisseria meningitidis PorB porin decreased or abrogated the mitochondrial damage induced by apoptotic stimuli. In addition, end stage determinants of apoptosis, including DNA breakdown, were diminished by PorB. Immunoprecipitation experiments revealed that PorB interacts with the mitochondrial porin VDAC (voltage-dependent anion channel). The mechanism of the antiapoptotic effect of neisserial porins could be explained by the protein–protein interaction of PorB with VDAC, similar to the interaction of VDAC with antiapoptotic Bcl-2 proteins, resulting in an enhancement of cell survival and continued activation of B cells.
Keywords: cell death, voltage-dependent anion channelVDAC, membrane potential, staurosporine
Neisserial porins, meningococcal PorA (class 1 protein) or PorB (class 2 or 3 proteins) or gonococcal protein IA or protein IB, are the major outer membrane protein of the pathogenic Neisseriaceae (1). They act as pores and are essential for organism survival. Interestingly, we and other investigators have demonstrated that these proteins act as immune stimulants and adjuvants (2–4) and can induce a T cell-dependent immune response against cell independent antigens (5–8). The mechanism of the adjuvant activity of neisserial porins recently has been elucidated, correlating with the porins' ability to up-regulate the expression of the costimulatory molecule, B7–2, on the surface of B cells (and possibly other antigen-presenting cells) (6, 8). Moreover, neisserial porins are potent B cell mitogens and act synergistically with CD40 or B cell receptor ligation to induce B cell proliferation and Ig secretion (6, 9).†
Stimulation of immune cells normally increases their susceptibility to activation-induced cell death or apoptosis (10). Neisserial porins activate B cells as a probable mechanism of their adjuvant activity. If the porins also increased the susceptibility of B cells to apoptosis, this obviously would attenuate their immunopotentiating ability. Therefore, we investigated the ability of neisserial porins to affect the susceptibility of various cell types to apoptosis to determine whether another mechanism of the porins' adjuvant activity is to prevent B cell (and/or antigen-presenting cell) apoptosis.
If neisserial porins decreased the susceptibility of immune cells to apoptosis, what could be the possible mechanisms? It has been demonstrated that mitochondria and mitochondrial factors are intimately connected to the process of apoptosis (11). The mechanism of action of certain apoptotic stimuli, such as Fas receptor ligation or drugs like staurosporine (STS), involves opening of the mitochondrial permeability transition pore (PT), dissipation of mitochondrial membrane potential (ΔΨm) (12), release of mitochondrial factors such as cytochrome c (13–15), apoptosis-inducing factor (16), or Apaf-1 (17) from the mitochondria into the cytosol. The release of cytochrome c initiates the apoptotic cascade through the activation of caspase-9 (18, 19).
Mitochondrial ΔΨm loss and cytochrome c release can be prevented by members of the bcl-2 gene family such as Bcl-xL or Bcl-2 (13, 20–22), which, similarly to neisserial porins, have been shown to insert into lipid vesicles or planar lipid bilayers and form ion-conducting channels in biological membranes (23, 24). Evidence suggests that the antiapoptotic effect of the Bcl-2 family members is mediated through a direct protein–protein interaction with voltage-dependent anionic channel (VDAC), a component of the mitochondrial pore (PT), thus stabilizing the pore (25). As this type of interaction also might be a mechanism of the potential neisserial porin antiapoptotic activity, in this study we investigated whether neisserial porins associate with mitochondria (and mitochondrial VDAC), preventing mitochondrial depolarization and cytochrome c release and determined whether the effects of neisserial porin on mitochondria are associated with an attenuation of apoptosis.
Materials and Methods
Cell Cultures.
Jurkat cells were cultured in RPMI 1640 medium containing 5% FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. CH12 RMC (a murine B cell line, a gift from R. Corley, Boston University School of Medicine) and murine splenic B cells were cultured in DMEM containing 8% FBS, 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 100 mM Hepes, and 10 mM β-mercaptoethanol. Murine splenic B cells were isolated from C3H/HeJ mice and C57 black mice per standard protocols (6). HeLa cells were cultured in DMEM containing 10% FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. (The CH-12 RMC B cell line shows a relatively high rate of spontaneous cell death, responsible for the increased background shown by the medium controls.)
Mitochondria Isolation and Western Blot.
Mitochondria were isolated as described (26). Purified mitochondrial and cytosolic fractions were subjected to 15% standard SDS/PAGE and transferred overnight on poly(vinylidene difluoride) membrane (Millipore). Blots were blocked for 1 h with 5% nonfat dry milk in 25 mM Tris (pH 8.0), 125 mM NaCl, 0.1% Tween 20, and 0.01% thimerosal and incubated overnight with anti-PorB rabbit polyclonal serum or the following mAbs: anti-cytochrome c (clone 7H8.2C12, PharMingen), anti-cytochrome oxidase (clone 20E8-C12, Molecular Probes), anti-PorB (National Institute for Biological Standards and Control, Potters Bar, Hertfordshire, United Kingdom), anti-VDAC (human clone 31HL, Calbiochem, La Jolla, CA) anti-LAMP1 and anti-Rab4 (Stressgen Biotechnology, Victoria BC, Canada), followed by horseradish peroxidase-labeled secondary antibody (Amersham Pharmacia). The immunoreactive bands were detected by enhanced chemiluminescence.
Immunoprecipitation.
Jurkat cells were incubated with medium or 10 μg/ml of PorB for 24 h. Mitochondria were isolated and sonicated in lysis buffer (10 mM Hepes, pH 7.4/142.5 mM KCl/5 mM MgCl2/1 mM EGTA/0.5% NP-40/17 μg/ml PMSF/8 μg/ml aprotinin/2 μg/ml leupeptin). Mitochondrial lysates were incubated with 10 μg of anti-PorB mAb per 106 cells with shaking for 1 h on ice. Protein-G agarose (Sigma) was added for 1 h at 4°C on a side to side rocker. Normal goat serum (1 mg/ml) (Sigma) was used to block nonspecific IgG binding sites. The agarose beads were centrifuged, washed with incubation buffer, resuspended in SDS/PAGE gel loading buffer, and analyzed by SDS/PAGE and Western blotting. PorB was purified and formed into detergent-free proteosomes as described (6, 27). As a control for nonspecific interaction of PorB with other mitochondrial components or membrane fragments, mitochondrial lysates were cleared of remaining membrane fragments by centrifugation for 1 h at 100,000 × g with an Airfuge (Beckman) and immunoprecipitation of PorB and VDAC from the supernatant was performed as described.
Induction of Apoptosis.
Jurkat and CH-12 RMC cells were incubated for 24 h with 1 μM STS (Sigma) for induction of apoptosis. A subset of these cultures was preincubated with 10 μg/ml of PorB for 24 h or 50 μM of the apoptosis inhibitor, Z-Val-Ala-Asp-(OMe)-CH2F (z-VAD-fmk) (Enzyme Systems Products, Dublin, CA) for 2 h before the addition of the apoptotic stimuli.
Flow Cytometric Analysis.
Isolated mitochondria were analyzed as follows. The mitochondria were incubated for 30 min on ice with anti-PorB polyclonal rabbit serum, or rabbit preimmune serum as a control, in the presence of 1 mg/ml of goat normal serum. After two washes with extraction buffer, anti-rabbit FITC-labeled IgG (Sigma) was added and the mitochondria was incubated for 15 min on ice, washed again, and immediately analyzed by flow cytometry on a FACScan flow cytometer by using cellquest acquisition and analysis software (Becton Dickinson). Mitochondrial mass (28) or ΔΨm (26) were analyzed in intact cells with nonyl acridine orange (NAO) and rhodamine 123 (rh123) (Molecular Probes) as follows. For NAO staining, treated cells were washed twice in ice-cold PBS, fixed with 80% ethanol at −20°C overnight, then incubated with 10 μM NAO for 15 min at room temperature and analyzed by flow cytometry. For rh123 staining, cells were incubated with 5 μg/ml of rh123 for 30 min at 37°C, then washed in cold PBS and immediately analyzed by flow cytometry. Gating was used to exclude cellular debris associated with necrosis and nonapoptotic cells.
Assessment of Cell Viability.
Aliquots of treated cells were stained with 0.2% trypan blue, incubated for 10 min at room temperature, and counted. A minimum of 100 cells were counted for each sample, and assays were performed in triplicate. The percentage of dead cells was calculated as a ratio between the number of dye-retaining cells (nonviable) and total number of cells per ml, as determined by using a hemacytometer. Data were analyzed by the Wilcoxon nonparametric rank sum test using sigmastat statistical software (SPSS, Chicago).
DNA Laddering and Propidium Iodide (PI) Staining of Hypodiploid DNA.
DNA laddering was measured by agarose gel electrophoresis as described (18). Briefly, cells were washed with PBS and resuspended in 20 μl of a solution containing 1% SDS, 0.5 mg/ml RNase A, and 5 μg/ml proteinase K and loaded on a 2% agarose gel. DNA was visualized by ethidium bromide fluorescence with a UV trans-illuminator. For PI staining, cells were washed, resuspended in 300 μl of PBS containing 2% FBS, and permeabilized with 750 μl of ice-cold ethanol for 10 min at 4°C. The cells then were resuspended in 300 μl of PBS containing 50 μg/ml PI and 0.5 mg/ml RNaseA, incubated for 20 min in the dark, washed once with PBS/FBS, and analyzed by flow cytometry. Cells were gated to exclude debris by using medium-treated control cells.
Results
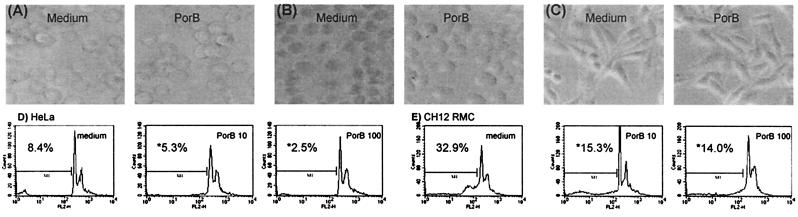
Incubation of Jurkat, HeLa, murine CH-12 RMC B cells, or murine splenic B cells with up to 100 μg/ml of PorB was not toxic for the cells and did not induce any visible modification in cellular morphology (Fig. 1 A–C). Hypodiploid DNA content of cells incubated with increasing concentrations of PorB for 24 h was measured by flow cytometric analysis of cells stained with PI. There was no increase of hypodiploid DNA content in PorB-treated cells as compared with untreated cells (Fig. 1 D and E), as opposed to increased hypodiploid DNA content after induction of apoptosis with STS (see Fig. 6). Moreover, the treatment with PorB did not affect the viability of the cells, as measured by trypan blue exclusion assay (Table 1).
Figure 1.
Phase microscopy of CH-12 RMC (A), Jurkat (B), and HeLa (C) cells incubated with medium alone or with 100 μg/ml PorB for 24 h. Incubation with PorB for 24 h does not induce increase of hypodiploid DNA content measured by flow cytometry of PI-stained HeLa (D) or CH-12 RMC (E) cells. The percentage of cells containing hypodiploid DNA was determined, as indicated. *, Not significant if compared with the medium control.
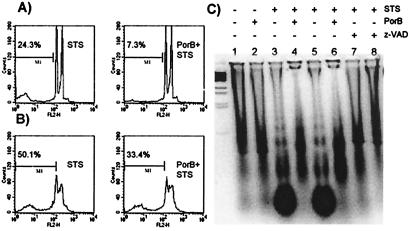
Figure 6.
Flow cytometry of PI fluorescence of Jurkat (A) and CH-12 RMC (B) cells incubated as described in the text. The percentage of cells containing hypodiploid DNA was measured by flow cytometry. The analysis gate has been drown accordingly to untreated samples (not shown). (C) DNA fragmentation pattern of CH-12 RMC cells incubated with: lane 1, medium alone; lane 2, 10 μg/ml PorB; lane 3, 1 μM STS for 4 h; lane 4, 10 μg/ml PorB for 24 h before STS incubation for 4 h; lane 5, 1 μM STS for 24 h; lane 6, 10 μg/ml PorB for 24 h before STS incubation for 24 h; lanes 7 and 8: 50 μM z-VAD-fmk before STS incubation for 4 and 24 h, respectively.
Table 1.
PorB does not induce cell death
| Cells | Medium | PorB, 10 μg/ml | PorB, 100 μg/ml |
|---|---|---|---|
| HeLa | 16.9 ± 4.6 | 17.6 ± 4.7 | 16.9 ± 0.8 |
| Jurkat | 5.4 ± 2.6 | 5.77 ± 2.9 | 5.4 ± 1.6 |
| CH-12 RMC | 49.2 ± 14.8 | 54.2 ± 12.4 | 51.2 ± 7.8 |
Cells were incubated for 24 h, and aliquots were collected and stained with trypan blue.
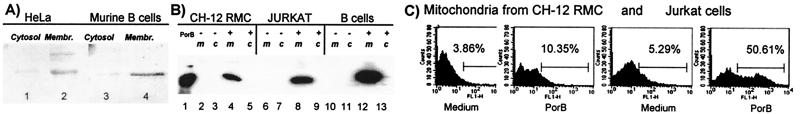
To investigate the fate of the porin in vitro, the subcellular localization of the porin was examined in several different types of mammalian cells. Cells were incubated for 24 h with 10 μg/ml of PorB and lysed, and total membrane and cytosolic fractions were obtained. Immunoblots with anti-PorB rabbit polyclonal serum revealed the presence of PorB in the membrane-containing fractions of all of the cells analyzed. Fig. 2A shows a representative immunoblot of fractions obtained from HeLa cells or murine B cells. To determine the further intracellular localization of the porin, fractions containing mitochondria, cytosol, or nuclei (plus other membrane compartments and unlysed cells) were obtained. The mitochondria were identified by Western blot with anti-cytochrome c mAb; anti-cytochrome oxidase IV mAb was used as a marker for mitochondria outer membrane integrity (26), and an immunoreactive band was detected only in association with the mitochondrial fractions, indicating that the mitochondrial outer membrane was not disrupted by the isolation procedure (data not shown). Anti-Lamp1 and anti-Rab4 mAbs also failed to detect lysosomal or endosomal membranes in the mitochondrial fractions (data not shown). After incubation of cells with 10 μg/ml of PorB, all fractions were analyzed by Western blot with anti-PorB mAb, and PorB was detected as a major band in the mitochondrial fractions (Fig. 2B). Traces of PorB also were detected in the nuclear fraction, which, however, as stated before, also contained other membrane compartments and a minor amount of unlysed cells (data not shown). The association of PorB with the mitochondria also was detected by flow cytometric analysis. Cells were incubated with medium or with 10 μg/ml of PorB for 24 h, and the mitochondria subsequently were isolated and incubated with anti-PorB polyclonal serum, followed by FITC-labeled anti-rabbit IgG. Histograms of fluorescence intensity revealed an increase in fluorescence associated with the mitochondria isolated from PorB-treated cells (Fig. 2C). Controls using preimmune rabbit serum failed to demonstrate a difference in fluorescence intensity between mitochondria from cells incubated with PorB or medium alone (data not shown).
Figure 2.
Cells were incubated with PorB as described in the text. (A) Cytosol and whole membranes from HeLa (lanes 1 and 2) and splenic murine B cells (lanes 3 and 4) were analyzed by Western blot with anti-PorB rabbit polyclonal serum. (B) Mitochondria (m) and cytosolic fractions (c) of CH-12 RMC (lanes 2–5), Jurkat (lanes 6–9), and splenic murine B cells (lanes 10–13) were analyzed with anti-PorB mAb. Purified PorB was used as molecular weight control (lane 1). (C) Mitochondria were isolated from CH-12 RMC and Jurkat cells, and the association of PorB with the purified mitochondria was detected by flow cytometry with anti-PorB rabbit polyclonal serum and FITC-labeled anti-rabbit second antibody. Preimmune rabbit serum was used as negative control for nonspecific binding, which was negligible.
The effect of PorB on ΔΨm and mitochondrial size (Table 2), after induction of apoptosis by STS treatment, was examined, and a protective effect of PorB treatment on these mitochondrial parameters was detected (Table 3 and Fig. 3). Mitochondrial size was measured by flow cytometry of whole cells stained with the fluorescent dye NAO, which specifically stains mitochondria in intact cells, and ΔΨm was measured by staining cells with rh123. Jurkat and CH12 RMC cells incubated with 10 μg/ml of PorB for 24 h and stained with NAO displayed comparable fluorescence to the cells incubated with medium alone (Table 2). Staining with an alternative mitochondrial size-specific dye, MitoTracker Green, also did not demonstrate any detectable changes in the mitochondrial volume after PorB treatment (data not shown). The variations of ΔΨm were measured as rh123 fluorescence of cells treated with PorB compared with cells incubated with medium alone, and as shown in Table 2, the ΔΨm was unaltered by PorB treatment.
Table 2.
PorB incubation does not affect mitochondrial size and ΔΨm
| Stain | Jurkat | CH-12 RMC |
|---|---|---|
| NAO | 1.03 ± 0.15 | 1.12 ± 0.04* |
| Rhodamine | 0.97 ± 0.1 | 0.96 ± 0.07 |
Mitochondrial mass and ΔΨm were measured by NAO and rh123 staining of whole cells, respectively. Values (±SDs) represent the mean fluorescence intensity ratios of PorB-treated cells relative to medium control cells. Each value has been averaged from three separate experiments.
No significant difference as compared to control.
Table 3.
PorB preincubation protects from mitochondrial size variations induced by STS
Mitochondrial size was measured by NAO staining of whole cells. Values (±SDs) represent the mean fluorescence intensity ratios relative to medium control cells. Each value has been averaged from three separate experiments. Statistical comparison between cells treated with STS alone or preincubated with PorB before the induction of apoptosis was performed by using the Wilcoxon nonparametric signed rank test.
No significant difference.
P < 0.0015.
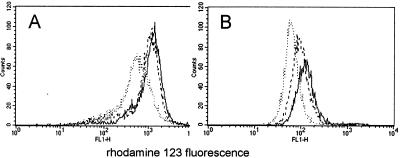
Figure 3.
Jurkat (A) and CH-12 RMC (B) cells incubated with medium or 10 μg/ml PorB for 24 h before the addition of 1 μM STS for an additional 24 h. ΔΨm was measured by flow cytometry of intact cells stained with rh123. Gating was used to exclude cellular debris. Solid lines, medium control cells. Dotted lines, STS alone. Dashed lines, PorB + STS.
The consequences of STS-mediated apoptosis on mitochondrial size and ΔΨm in the presence of PorB then were examined. Typically, cells incubated with STS go through several alterations before cell death occurs, including mitochondrial volume alterations and membrane depolarization, followed by release of cytochrome c into the cytosol, DNA fragmentation, and more (19, 26, 29). Mitochondrial volume changes induced by treatment of Jurkat or CH-12 RMC cells with 1 μM STS for 24 h were measured by flow cytometry of NAO-stained cells. The NAO fluorescence of STS-treated Jurkat cells was only slightly decreased as compared with untreated cells; CH-12 RMC cells, on the contrary, showed a marked loss of mitochondrial volume after the treatment (probably because of higher condensation of mitochondria and lower cardiolipin levels), suggesting that the volume variations of mitochondria were perhaps differently affected by STS treatment, depending on the cell type (Table 3). Preincubation with 10 μg/ml of PorB for 24 h before the addition of STS partially protected CH-12 RMC cells from loss of mitochondrial volume (Table 3). The ΔΨm variations induced in Jurkat and CH-12 RMC cells by STS were determined. Incubation with STS as above induced dissipation of ΔΨm; pretreatment of cells with PorB before the addition of STS decreased the loss of ΔΨm (Fig. 3). Primary murine B cells ΔΨm also was examined and demonstrated similar behavior as compared with the CH12 RMC cell line (data not shown).
Cytochrome c release into the cytosol, which is important for generation of downstream apoptotic events (13), has been reported as a consequence of STS treatment (13, 26), likely because of the opening of the mitochondrial PT, which follows the ΔΨm loss. Therefore, the ability of PorB to prevent cytochrome c release induced by STS was examined. Cells were incubated with medium alone or with 10 μg/ml of PorB for 24 h, and no release of cytochrome c into the cytosol was detected (Fig. 4A). Mitochondria and cytosolic fractions of cells incubated with PorB before the addition of 1 μM STS (Fig. 4B) revealed a protection from release of cytochrome c into the cytosol. The caspase inhibitor z-VAD-(fmk) (which protects cells from apoptosis by blocking caspase-9 activation but does not prevent mitochondrial cytochrome c release into the cytosol) was used as control.
Figure 4.
Mitochondria (mit) and cytosolic fractions (cyt) from CH-12 RMC cells treated with: medium alone or 10 μg/ml PorB (A) and medium, PorB, or 50 μM z-VAD-fmk for 24 h before addition of STS (B), were analyzed by Western blot with anti-cytochrome c mAb.
The possibility that PorB interacts with the mitochondrial PT pore complex was examined by immunoprecipitation experiments. Jurkat cells were incubated with medium or with 10 μg/ml of PorB for 24 h, and the mitochondria were isolated and lysed in the presence of detergent, to dissolve mitochondrial membrane fragments and extract the outer membrane proteins. The lysates were incubated with anti-PorB mAb for immunoprecipitation of PorB and the proteins with which it may associate. Western blot analysis of the immunoprecipitates with anti-PorB antibody or anti-VDAC antibody demonstrated that VDAC coimmunoprecipitated with PorB (Fig. 5). To exclude the possibility of an interaction of PorB with other mitochondrial compartments, fragments of mitochondrial membrane, or unlysed mitochondria, the lysates were cleared by a high-speed centrifugation step. Western blot analysis after immunoprecipitation as described above confirmed the coimmunoprecipitation of VDAC with PorB (data not shown).
Figure 5.
Jurkat cells were incubated with medium (lanes 1) or 10 μg/ml PorB (lanes 2) for 24 h, and mitochondria were isolated and immunoprecipitated with anti-PorB mAb. The immune complexes were analyzed by Western blotting using anti-PorB antibody or anti-human VDAC antibody as indicated.
The potential protective effect of PorB on apoptotic cell death was indirectly measured by trypan blue exclusion assay. Jurkat cells and CH12 RMC cells were incubated with 1 μM STS for induction of apoptosis or with medium alone. The number of cells retaining the dye per ml and the number of total cells per ml were determined in each sample to calculate the percent of dead cells (Table 4). When the cells were incubated with PorB before the apoptotic stimuli, the percentage of dead cells decreased after STS treatment. The caspase inhibitor z-VAD-fmk was used as a control.
Table 4.
PorB protects from cell death induced by STS
| Treatment | Jurkat | CH-12 RMC |
|---|---|---|
| STS | 22.2 ± 12.4* | 80.2 ± 14.8† |
| PorB + STS | 15 ± 8.3 | 65.6 ± 13.0 |
| z-VAD-fmk + STS | 11.2 ± 9.4 | 55.6 ± 6.4 |
Cells were incubated as described in the text. z-VAD-fmk (50 μM) was used as inhibitor of cell death. Statistical comparison between cells treated with STS alone or preincubated with PorB before the induction of apoptosis was performed by using the Wilcoxon nonparametric signed rank test.
P < 0.03.
P < 0.006.
PorB protection from DNA degradation after STS treatment was evaluated by two distinct methods: (i) measuring hypodiploid DNA content by PI staining and flow cytometry and (ii) examining the extent of DNA laddering on agarose gel. Jurkat or CH-12 RMC cells were incubated with medium alone, PorB, and/or STS and subsequently permeabilized and stained with PI. When cells were incubated with STS, an increase in hypodiploid DNA content was detectable; if the cells were preincubated with PorB before the addition of STS, a marked decrease in the percentage of hypodiploid DNA content was seen (Fig. 6 A and B). Gates were drawn accordingly to prevent analysis of cellular debris by using the medium-treated cells (not shown in Fig. 6). CH-12 RMC cells demonstrated a minimal background extent of hypodiploid DNA because of a higher rate of spontaneous cell death as compared with Jurkat cells (refer to Fig. 1).
DNA laddering was measured after incubation of cells with PorB for 24 h before the addition of STS for 4 h or 24 h. Cells were lysed and digested with RNase A and proteinase K directly before being loaded on agarose gel. Medium- or PorB-treated cells revealed identical minimal DNA degradation, whereas DNA laddering was manifest after 4 h (Fig. 6C, lane 3) or 24 h (Fig. 6C, lane 5) of STS incubation, as indicated by low molecular weight DNA fragments (Fig. 6C). Preincubation with PorB (Fig. 6C, lanes 4 and 6) inhibited formation of apoptosis-associated DNA breakdown products as indicated by the decrease of DNA laddering. As a positive control, 50 μM z-VAD-fmk prevented STS-induced DNA fragmentation.
Discussion
We examined whether the porin's ability to activate B cells affects the cells' susceptibility to activation-induced cell death and whether the porins themselves can directly protect cells from apoptosis. This is potentially another mechanism of the porin's immunopotentiating ability. The porin's ability to affect B cell function clearly requires a priori an active interaction with the lymphocyte itself. There is much preliminary evidence that neisserial porins can directly interact with artificial planar lipid bilayers (41) and eukaryotic cell membranes (30, 31) and form aqueous transmembrane channels for the transport of solutes and macromolecules across the outer membrane. In addition, they can form voltage-gated pores at low membrane potential (32, 33). These pores are regulated by ATP and GTP with a gating mechanism that modulates the pore size and ion selectivity (similar to mitochondrial VDAC) (34), when they insert in mammalian cells, potentially contributing to the pathogenicity of the microorganism (35). Interestingly, our findings show that after incubation of different types of cells, such as lymphoid cells and epithelial cells with purified PorB, the porin primarily localizes into the mitochondrial compartment. The association of PorB with mitochondria results in a stabilization of mitochondrial membrane and enhanced cell survival when an apoptotic stimulus, such as STS, is provided.
The interaction of porin with target cells was examined by using epithelial and lymphoid cell lines. Incubation with high concentrations of meningococcal PorB for up to 72 h did not induce cellular changes consistent with necrosis or apoptosis. Our findings are in contrast to what has been published by Müller et al. (36), who describe induction of apoptosis in HeLa cells incubated with gonococcal porin (protein IB). The discrepancies between our data and Müller et al.'s data could be caused by the fact that (i) our studies have been performed mostly on lymphoid cells in medium supplemented with growth factors (FBS) rather than epithelial cells in serum-free culture (a condition that may render cells more sensitive to apoptosis; refs. 37 and 38); (ii) our porins were purified by using different protocols (9, 27), with a final concentration of detergent lower than that obtained by Müller et al., and (iii) different neisserial porins were used. Incubation of HeLa cells with gonococcal protein IB or PorB purified according to our protocol did not induce cell death even in serum-depleted culture (data not shown), excluding the possibility of any toxic effect of neisserial porins on epithelial cells in our system.
The structure of neisserial porins reveals a trimeric β-pleated barrel with a high percentage (36%) of β-sheet secondary structures (39, 40). Mitochondrial porins, or VDACs, also are characterized by a similar β-barrel conformation and share functional characteristics with neisserial porins, including regulation of the pore size by nucleotides and the ability of “autodirected” insertion into phospholipid membranes (34). VDACs contribute to the regulation of metabolite flux through the mitochondrial membrane and are part of a larger structure (PT). We investigated whether neisserial porins can enter eukaryotic cells and interact with the mitochondria, based on the structural and functional similarities of neisserial porins and mitochondrial porins and the suggestive evidence that neisserial porins must interact with lymphocytes to induce their immunopotentiating effect. It has been shown that neisserial porins vectorially translocate into artificial membranes and target cells (41), and it has been proposed that they might bind or insert into the membrane of epithelial cells at the sites of close contact between bacteria and cells during the infection (42, 43). The mechanism of translocation of the porins into the target cells is still poorly characterized, and thus there is no evidence of a specific receptor-mediated event accounting for porin. We investigated the intracellular localization of PorB in several different cell types and demonstrated the association of PorB with the mitochondria. The integrity of the mitochondrial outer membrane was ascertained with anti-cytochrome IV oxidase antibody. Moreover, anti-LAMP1 or anti-Rab4 antibodies failed to detect immunoreactive bands by Western blotting, indicating the absence of endosomes or lysosomes copurified with the mitochondria. Trace amounts of PorB also was detected in the fraction containing nuclei, other membranes, and unlysed cells obtained during the mitochondria isolation. It might be argued PorB also might interact with cellular membranes, but the major interaction demonstrated is with the mitochondria. Anti-PorB antibody was used for an immunoprecipitation experiment of mitochondria isolated from cells treated with PorB, and the association of the porin with the mitochondria was confirmed. The association with mitochondria did not appear to be a characteristic unique for Neisseria meningitidis PorB; in fact, Neisseria gonorrhoea protein IB also was detected in the mitochondrial fractions after 24 h of incubation with Jurkat or CH12 RMC cells (data not shown).
Mitochondrial changes have been observed in several models of apoptosis induced by different stimuli, including Fas receptor binding (44), drugs (45), UV irradiation (15), serum starvation (38), or expression of proapoptotic members of the bcl-2 family such as Bax or Bak (19, 46). Mitochondria may be subjected to volume and size changes after an apoptotic insult, including swelling at an early stage during apoptosis followed by condensation of the matrix and cell shrinkage (11, 12). Relative changes in mitochondrial volume were measured by flow cytometric analysis of NAO fluorescence. There were no detectable differences of NAO fluorescence ratios between untreated cells or PorB-treated cells as opposed to the obvious differences between untreated cells and cells treated with apoptotic stimuli or differences in fluorescence as detected by another mitochondria-specific dye, Mitotracker Green (data not shown). These data indicate that PorB incubation did not elicit condensation of the mitochondrial matrix indicative of apoptosis.
Loss of ΔΨm across the inner mitochondrial membrane has been proposed as a hallmark of apoptosis (11). If the interaction of PorB with the mitochondria would affect the apoptotic process, variations of parameters, such as the ΔΨm or mitochondrial size, might be expected. Rh123 is a specific dye used to measure the electrochemical gradient of the mitochondrial membrane (47). The rh123 fluorescence intensity of PorB-treated and medium control cells were superimposable, indicating that PorB did not induce ΔΨm depolarization in Jurkat cells and CH-12 RMC cells or in murine splenic B cells and HeLa cells (data not shown). Other markers of apoptosis, including cell morphology (i.e., shape, adherence, or retraction of pseudopodia), trypan blue exclusion, and DNA breakdown, indicated that the interaction of N. meningitidis PorB, as stated previously, was not recognized as an apoptosis-inducing stimulus. STS treatment can result in a decrease of mitochondrial volume and depolarization of the ΔΨm, which normally would be seen with ongoing apoptosis (13, 26, 29). The effect of PorB on the mitochondrial alterations induced by STS was examined and revealed a consistent protection against both mitochondrial volume change and ΔΨm loss. Mitochondrial volume changes after STS treatment was partially abolished in CH-12 RMC cells, whereas the volume of mitochondria in Jurkat cells appeared not to be altered by the treatment, possibly because of a different sensitivity of these cells to the drug. STS-induced ΔΨm loss in Jurkat cells and CH-12 RMC cells was reduced as result of PorB preincubation, as measured by rh123 fluorescence, as well as in splenic murine B cells and HeLa cells (data not shown).
Modification of the ΔΨm may induce opening of the mitochondrial permeability transition pore, followed by cytochrome c release from the mitochondrial intermembrane space into the cytosol (13). The release of cytochrome c into the cytosol appears to initiate the apoptotic cascade through the activation of caspase-9 (48) and STS-induced cytochrome c translocation into the cytosol has been described (18). We therefore investigated the effect of PorB on STS-induced cytochrome c release. PorB treatment by itself did not induce cytochrome c release. In fact, this treatment was effective in preventing or reducing cytochrome c release in the cytosol of cells treated with STS. This result suggests that the association of PorB with mitochondria might not only be involved in the electrical stabilization of the outer membrane, but also play a role in the regulation of the passage of large molecules through the mitochondrial membrane.
Members of the Bcl-2 family like Bcl-2 or Bcl-xL, directly participate in the regulation of mitochondrial PT gating occurring after induction of apoptosis (13). Structural similarities between pore-forming domains of some toxins including diphtheria toxin and related colicins and members of the bcl-2 gene family have been described (22), and they display an in vitro ion-channel forming activity when added to synthetic membranes (23, 24). The mitochondrial porin, VDAC, part of the PT, recently has been reported to interact with the antiapoptotic protein Bcl-xL, and their association appears to elicit the closure of the mitochondrial PT, through which cytochrome c could escape during apoptosis (25). A similar interaction between PorB and VDAC affecting the opening and closing of the VDAC pore could be the mechanism of the antiapoptotic effect of neisserial porins. To examine this hypothesis, mitochondria from cells treated with PorB were immunoprecipitated with either anti-PorB antibody or anti-VDAC antibody (data not shown). Analysis of the immunoprecipitates by the two different antibodies revealed the presence of both PorB and VDAC by Western blot, indicating that VDAC was coimmunoprecipitated with PorB, suggesting that an interaction of PorB with the mitochondrial membrane might take place at the PT site, directly or indirectly with VDAC.
Induction of apoptosis presupposes induction of cellular death. Cell viability, and indirectly, its opposite, cell death, can be measured in a population by the trypan blue exclusion assay. This permeable dye rapidly diffuses in all of the cells and it is actively expelled by live cells. Viability of cells treated with STS alone or with PorB before the addition of STS was measured by trypan blue staining and correlated with the protective effect of PorB shown for the mitochondria and DNA integrity. DNA breakdown is one of the hallmarks of apoptosis. Apoptotic hypodiploid DNA content was measured after STS treatment and was reduced when Jurkat, CH-12 RMC cells, or murine splenic B cells (data not shown) were preincubated with PorB before induction of apoptosis. DNA degradation also is characterized by laddering of small-size fragments on agarose gels. Cells incubated with medium or with PorB alone demonstrated comparable minimal laddering, whereas DNA from STS-treated cells exhibited a typical degradation because of apoptosis, which was not detected when the cells were pretreated with PorB before incubation with STS. The mechanism of protection from DNA fragmentation is not clear, but it might be likely the result of a modulation of the intensity of the intracellular signals originating from the mitochondria that lead to apoptosis.
In conclusion, this work demonstrates that the interaction of mammalian cells and neisserial porins in vitro involves the intracellular translocation of the porin, followed by localization into the mitochondrial compartment and interaction with a protein component of the mitochondrial PT, VDAC porin. The result of this association is a stabilization of mitochondrial membrane and an increase in cell survival when an apoptotic stimulus, such as STS, is provided. In the light of our evidence of the interaction of PorB with VDAC, and the interaction of VDAC with Bcl-2 family members (25), we could speculate that PorB might be able to modulate the permeability transition pores in a fashion similar to Bcl-2 family members. More data will be needed to prove that PorB interaction with mitochondria in vitro occurs in a manner similar to Bcl-2 family members. The relationship of this finding to the immunopotentiating ability of neisserial porins is unclear, but it is probable that preventing activation-induced cell death or apoptosis of B cells stimulated by the porin enhances the B cells survival, and therefore, enhances the immune response with which these B cells are involved. Therefore, inhibition of immune cells apoptosis by neisserial porins could be an inherent mechanism of their immunopotentiating ability.
Acknowledgments
We thank Michael Copass and Dr. Marco Colombini for valuable discussion during the course of our studies and Dr. Michael Starnbach and Dr. Dave Carroll for critical readings of the manuscript. This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant AI40944.
Abbreviations
- VDAC
voltage-dependent anion channel
- STS
staurosporine
- PT
permeability transition pore
- ΔΨm
mitochondrial membrane potential
- NAO
nonyl acridine orange
- PI
propidium iodide
- rh123
rhodamine 123
Footnotes
Portions of this work were presented at the 11th International Pathogenic Neisseria Conference, November, 1–6, 1998, Nice, France.
References
- 1.Blake M S, Gotschlich E C. In: Bacterial Outer Membranes as Model Systems. Inouye M, editor. New York: Wiley; 1986. pp. 377–400. [Google Scholar]
- 2.Lowell G H, Ballou W R, Smith L F, Wirtz R A, Zollinger W D, Hockmeyer W T. Science. 1988;240:800–802. doi: 10.1126/science.2452484. [DOI] [PubMed] [Google Scholar]
- 3.Wetzler L M. In: Pathobiology and Immunobiology of Neisseriaceae. Conde-Glez C J, Morse S A, Rice P A, Sparling P F, Calderón E, editors. Cuernavaca, Morelos, Mexico: Instituto Nacional de Salud Pública; 1994. pp. 127–134. [Google Scholar]
- 4.Livingston P O, Calves M J, Helling F, Zollinger W D, Blake M S, Lowell G H. Vaccine. 1993;11:1199–1204. doi: 10.1016/0264-410x(93)90043-w. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly J J, Deck R R, Liu M A. J Immunol. 1990;145:3071–3079. [PubMed] [Google Scholar]
- 6.Wetzler L M, Ho Y, Reiser H. J Exp Med. 1996;183:1151–1159. doi: 10.1084/jem.183.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fusco P C, Michon F, Laude-Sharp M, Minetti C A, Huang C H, Heron I, Blake M S. Vaccine. 1998;16:1842–1849. doi: 10.1016/s0264-410x(98)00174-1. [DOI] [PubMed] [Google Scholar]
- 8.Mackinnon F G, Ho Y, Blake M S, Michon F, Chandraker A, Sayegh M H, Wetzler L M. J Infect Dis. 1999;180:755–761. doi: 10.1086/314966. [DOI] [PubMed] [Google Scholar]
- 9.Snapper C M, Rosas F R, Kehry M R, Mond J J, Wetzler L M. Infect Immun. 1997;65:3203–3208. doi: 10.1128/iai.65.8.3203-3208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott D W, Grdina T, Shi Y. J Immunol. 1996;156:2352–2356. [PubMed] [Google Scholar]
- 11.Kroemer G, Dallaporta B, Resche-Rigon M. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 12.Petit P X, Goubern M, Diolez P, Susin S A, Zamzami N, Kroemer G. FEBS Lett. 1998;426:111–116. doi: 10.1016/s0014-5793(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 13.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 14.Stridh H, Kimland M, Jones D P, Orrenius S, Hampton M B. FEBS Lett. 1998;429:351–355. doi: 10.1016/s0014-5793(98)00630-9. [DOI] [PubMed] [Google Scholar]
- 15.Bossy-Wetzel E, Newmeyer D D, Green D R. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Nature (London) 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 17.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 18.Wolf C M, Eastman A. Exp Cell Res. 1999;247:505–513. doi: 10.1006/excr.1998.4380. [DOI] [PubMed] [Google Scholar]
- 19.Finucane D M, Bossy-Wetzel E, Waterhouse N J, Cotter T G, Green D R. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 21.Schneider T J, Grillot D, Foote L C, Nunez G E, Rothstein T L. J Immunol. 1997;159:4834–4839. [PubMed] [Google Scholar]
- 22.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S L, et al. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 23.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 26.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 27.Wetzler L M, Blake M S, Gotschlich E C. In: UCLA Symposia on Molecular and Cellular Biology: Technological Advances in Vaccine Development. Lasky L, editor. New York: Liss; 1988. pp. 21–34. [Google Scholar]
- 28.Camilleri-Broet S, Vanderwerff H, Caldwell E, Hockenbery D. Exp Cell Res. 1998;239:277–292. doi: 10.1006/excr.1997.3899. [DOI] [PubMed] [Google Scholar]
- 29.Heiskanen K M, Bhat M B, Wang H W, Ma J, Nieminen A L. J Biol Chem. 1999;274:5654–5658. doi: 10.1074/jbc.274.9.5654. [DOI] [PubMed] [Google Scholar]
- 30.Weel J F, Hopman C T, van Putten J P. J Exp Med. 1991;174:705–715. doi: 10.1084/jem.174.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Putten J P, Duensing T D, Carlson J. J Exp Med. 1998;188:941–952. doi: 10.1084/jem.188.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauro A, Blake M, Labarca P. Proc Natl Acad Sci USA. 1988;85:1071–1075. doi: 10.1073/pnas.85.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, Minetti C A, Blake M S, Colombini M. Biochim Biophys Acta. 1998;1370:289–298. doi: 10.1016/s0005-2736(97)00279-4. [DOI] [PubMed] [Google Scholar]
- 34.Colombini M, Blachly-Dyson E, Forte M. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- 35.Rudel T, Schmid A, Benz R, Kolb H A, Lang F, Meyer T F. Cell. 1996;85:391–402. doi: 10.1016/s0092-8674(00)81117-4. [DOI] [PubMed] [Google Scholar]
- 36.Muller A, Gunther D, Naumann M, Meyer T F, Rudel T. EMBO J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulkarni G V, McCulloch C A. J Cell Sci. 1994;107:1169–1179. doi: 10.1242/jcs.107.5.1169. [DOI] [PubMed] [Google Scholar]
- 38.Simm A, Bertsch G, Frank H, Zimmermann U, Hoppe J. J Cell Sci. 1997;110:819–828. doi: 10.1242/jcs.110.7.819. [DOI] [PubMed] [Google Scholar]
- 39.Derrick J P, Urwin R, Suker J, Feavers I M, Maiden M C. Infect Immun. 1999;67:2406–2413. doi: 10.1128/iai.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minetti C A, Tai J Y, Blake M S, Pullen J K, Liang S M, Remeta D P. J Biol Chem. 1997;272:10710–10720. doi: 10.1074/jbc.272.16.10710. [DOI] [PubMed] [Google Scholar]
- 41.Lynch E C, Blake M S, Gotschlich E C, Mauro A. Biophys J. 1984;45:104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGee Z A, Johnson A P, Taylor-Robinson D. J Infect Dis. 1981;143:413–421. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- 43.Virji M, Alexandrescu C, Ferguson D J, Saunders J R, Moxon E R. Mol Microbiol. 1992;6:1271–1279. doi: 10.1111/j.1365-2958.1992.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 44.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boesen-de Cock J G, Tepper A D, de Vries E, van Blitterswijk W J, Borst J. J Biol Chem. 1999;274:14255–14261. doi: 10.1074/jbc.274.20.14255. [DOI] [PubMed] [Google Scholar]
- 46.Narita M, Shimizu S, Ito T, Chittenden T, Lutz R J, Matsuda H, Tsujimoto Y. Proc Natl Acad Sci USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemasters J J, DiGuiseppi J, Nieminen A L, Herman B. Nature (London) 1987;325:78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- 48.Sun X M, MacFarlane M, Zhuang J, Wolf B B, Green D R, Cohen G M. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]