Abstract
Deficiency of argininosuccinate synthetase (ASS) causes citrullinemia in human beings. Type II citrullinemia is found in most patients with adult-onset citrullinemia in Japan, and ASS deficiency is found specifically in the liver. Previous studies have shown that the decrease of hepatic ASS activity is caused by a decrease in enzyme protein with normal kinetic properties and that there were no apparent abnormalities in the amount, translational activity, and gross structure of hepatic ASS mRNA. In the present work, we show by sequencing analysis that there was no mutation in the ASS mRNA from two patients with type II citrullinemia. We also report RFLP analysis of a consanguineous family with type II citrullinemia, by using three DNA polymorphisms located within the ASS gene locus. In spite of having consanguineous parents, the patient was not a homozygous haplotype for the ASS gene. The RFLP analysis of 16 affected patients from consanguineous parents showed that 5 of 16 patients had the heterozygous pattern for one of the three DNA probes and that the frequency of the heterozygous haplotype was not different from the control frequency. These results suggest that the primary defect of type II citrullinemia is not within the ASS gene locus.
Full text
PDF

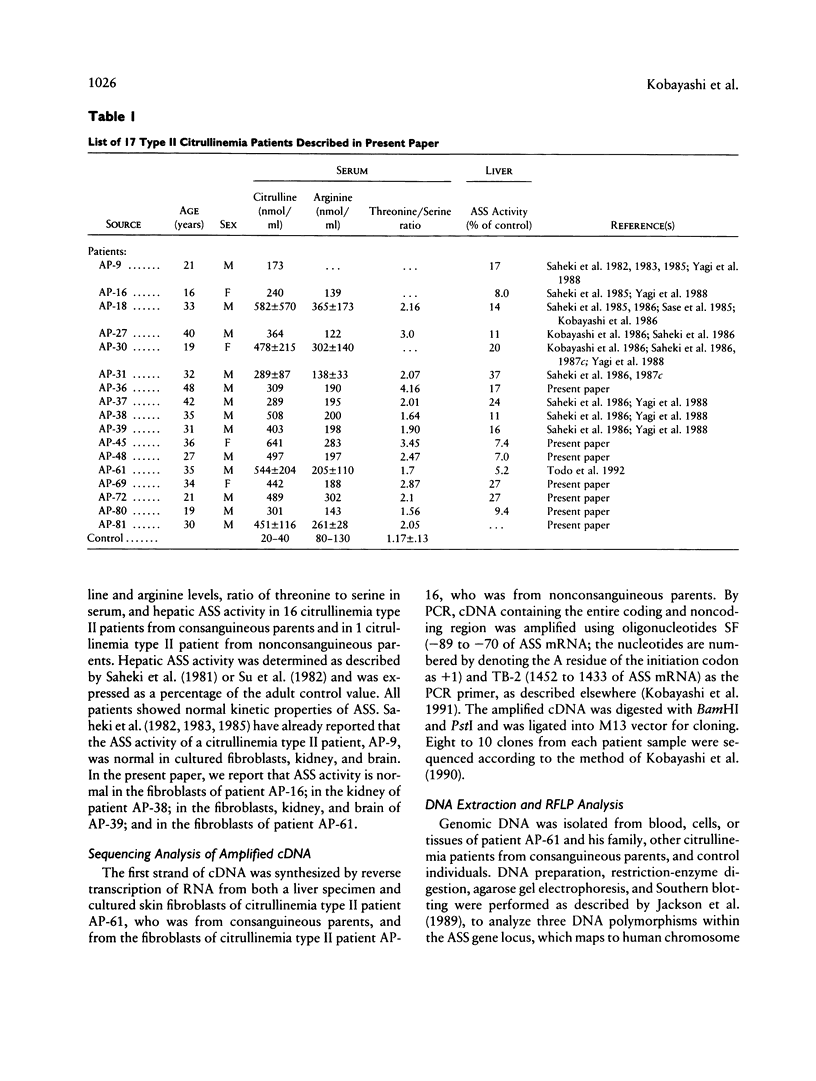
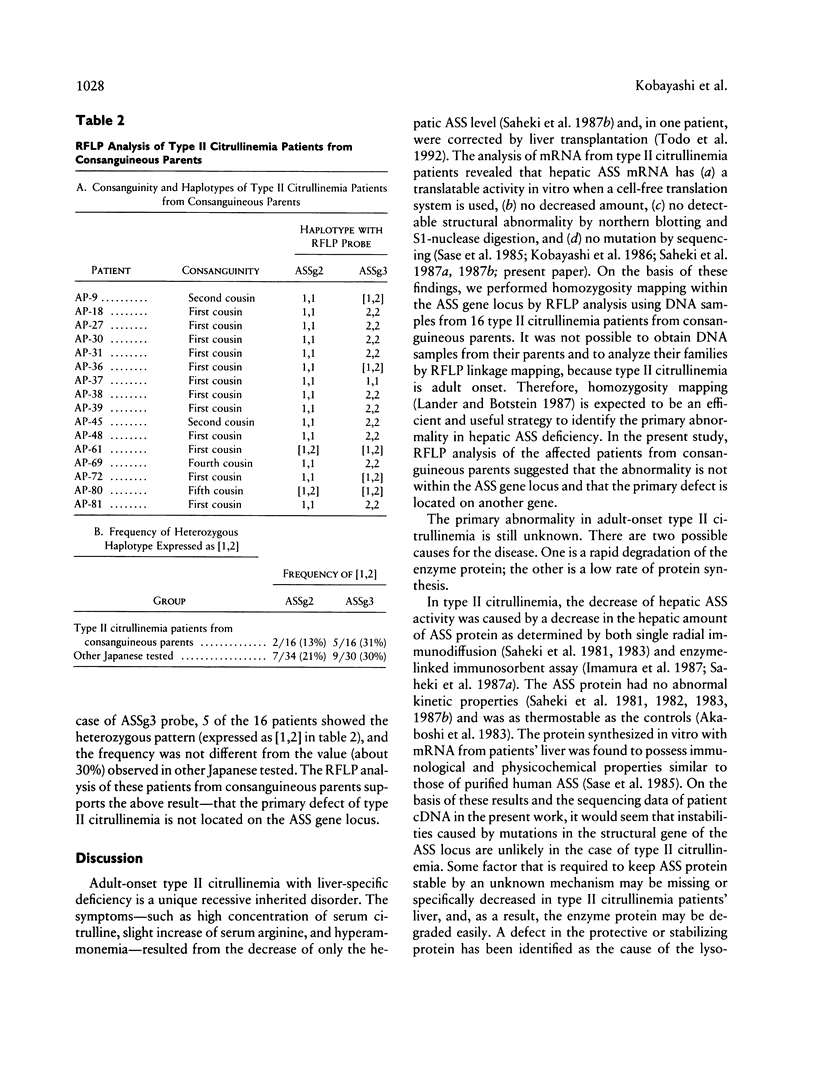


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaboshi I., Endo F., Matsuda I., Saheki T. Kinetic analysis of argininosuccinate synthetase in a variant form of citrullinaemia. J Inherit Metab Dis. 1983;6(1):36–39. doi: 10.1007/BF02391191. [DOI] [PubMed] [Google Scholar]
- Beaudet A. L., O'Brien W. E., Bock H. G., Freytag S. O., Su T. S. The human argininosuccinate synthetase locus and citrullinemia. Adv Hum Genet. 1986;15:161-96, 291-2. doi: 10.1007/978-1-4615-8356-1_3. [DOI] [PubMed] [Google Scholar]
- Dahlberg G. Inbreeding in Man. Genetics. 1929 Sep;14(5):421–454. doi: 10.1093/genetics/14.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen A. T., Verheijen F. W., Galjaard H. The relation between human lysosomal beta-galactosidase and its protective protein. J Biol Chem. 1983 Oct 25;258(20):12143–12146. [PubMed] [Google Scholar]
- Imaizumi Y., Shinozaki N., Aoki A. Inbreeding in Japan: Results of a nation-wide study. Jinrui Idengaku Zasshi. 1975 Sep;20(2):91–107. [PubMed] [Google Scholar]
- Imamura Y., Kobayashi K., Yamashita T., Saheki T., Ichiki H., Hashida S., Ishikawa E. Clinical application of enzyme immunoassay in the analysis of citrullinemia. Clin Chim Acta. 1987 Apr 30;164(2):201–208. doi: 10.1016/0009-8981(87)90071-4. [DOI] [PubMed] [Google Scholar]
- Jackson M. J., Kobayashi K., Beaudet A. L., O'Brien W. E. Analysis of deletions at the human argininosuccinate synthetase locus. Mol Biol Med. 1989 Apr;6(2):179–186. [PubMed] [Google Scholar]
- Kobayashi K., Jackson M. J., Tick D. B., O'Brien W. E., Beaudet A. L. Heterogeneity of mutations in argininosuccinate synthetase causing human citrullinemia. J Biol Chem. 1990 Jul 5;265(19):11361–11367. [PubMed] [Google Scholar]
- Kobayashi K., Rosenbloom C., Beaudet A. L., O'Brien W. E. Additional mutations in argininosuccinate synthetase causing citrullinemia. Mol Biol Med. 1991 Feb;8(1):95–100. [PubMed] [Google Scholar]
- Kobayashi K., Saheki T., Imamura Y., Noda T., Inoue I., Matuo S., Hagihara S., Nomiyama H., Jinno Y., Shimada K. Messenger RNA coding for argininosuccinate synthetase in citrullinemia. Am J Hum Genet. 1986 May;38(5):667–680. [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987 Jun 19;236(4808):1567–1570. doi: 10.1126/science.2884728. [DOI] [PubMed] [Google Scholar]
- Nanba E., Tsuji A., Omura K., Suzuki Y. Galactosialidosis: a direct evidence that a 46-kilodalton protein restores deficient enzyme activities in fibroblasts. Biochem Biophys Res Commun. 1987 Apr 14;144(1):138–142. doi: 10.1016/s0006-291x(87)80486-2. [DOI] [PubMed] [Google Scholar]
- Northrup H., Lathrop M., Lu S. Y., Daiger S. P., Beaudet A. L., O'Brien W. E. Multilocus linkage analysis with the human argininosuccinate synthetase gene. Genomics. 1989 Oct;5(3):442–444. doi: 10.1016/0888-7543(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Palmeri S., Hoogeveen A. T., Verheijen F. W., Galjaard H. Galactosialidosis: molecular heterogeneity among distinct clinical phenotypes. Am J Hum Genet. 1986 Feb;38(2):137–148. [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Kobayashi K., Ichiki H., Matuo S., Tatsuno M., Imamura Y., Inoue I., Noda T., Hagihara S. Molecular basis of enzyme abnormalities in urea cycle disorders. With special reference to citrullinemia and argininosuccinic aciduria. Enzyme. 1987;38(1-4):227–232. doi: 10.1159/000469209. [DOI] [PubMed] [Google Scholar]
- Saheki T., Kobayashi K., Inoue I. Hereditary disorders of the urea cycle in man: biochemical and molecular approaches. Rev Physiol Biochem Pharmacol. 1987;108:21–68. doi: 10.1007/BFb0034071. [DOI] [PubMed] [Google Scholar]
- Saheki T., Kobayashi K., Inoue I., Matuo S., Hagihara S., Noda T. Increased urinary excretion of argininosuccinate in type II citrullinemia. Clin Chim Acta. 1987 Dec;170(2-3):297–304. doi: 10.1016/0009-8981(87)90140-9. [DOI] [PubMed] [Google Scholar]
- Saheki T., Ueda A., Hosoya M., Kusumi K., Takada S., Tsuda M., Katsunuma T. Qualitative and quantitative abnormalities of argininosuccinate synthetase in citrullinemia. Clin Chim Acta. 1981 Feb 5;109(3):325–335. doi: 10.1016/0009-8981(81)90318-1. [DOI] [PubMed] [Google Scholar]
- Saheki T., Ueda A., Hosoya M., Sase M., Nakano K., Katsunuma T. Enzymatic analysis of citrullinemia (12 cases) in Japan. Adv Exp Med Biol. 1982;153:63–76. doi: 10.1007/978-1-4757-6903-6_9. [DOI] [PubMed] [Google Scholar]
- Saheki T., Ueda A., Iizima K., Yamada N., Kobayashi K., Takahashi K., Katsunuma T. Argininosuccinate synthetase activity in cultured skin fibroblasts of citrullinemic patients. Clin Chim Acta. 1982 Jan 5;118(1):93–97. doi: 10.1016/0009-8981(82)90230-3. [DOI] [PubMed] [Google Scholar]
- Sase M., Kobayashi K., Imamura Y., Saheki T., Nakano K., Miura S., Mori M. Level of translatable messenger RNA coding for argininosuccinate synthetase in the liver of the patients with quantitative-type citrullinemia. Hum Genet. 1985;69(2):130–134. doi: 10.1007/BF00293282. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., Beaudet A. L., O'Brien W. E. Molecular analysis of argininosuccinate synthetase deficiency in human fibroblasts. J Clin Invest. 1982 Dec;70(6):1334–1339. doi: 10.1172/JCI110736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. S., Lin L. H. Analysis of a splice acceptor site mutation which produces multiple splicing abnormalities in the human argininosuccinate synthetase locus. J Biol Chem. 1990 Nov 15;265(32):19716–19720. [PubMed] [Google Scholar]
- Todo S., Starzl T. E., Tzakis A., Benkov K. J., Kalousek F., Saheki T., Tanikawa K., Fenton W. A. Orthotopic liver transplantation for urea cycle enzyme deficiency. Hepatology. 1992 Mar;15(3):419–422. doi: 10.1002/hep.1840150311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Saheki T., Imamura Y., Kobayashi K., Sase M., Nakano K., Matuo S., Inoue I., Hagihara S., Noda T. The heterogeneous distribution of argininosuccinate synthetase in the liver of type II citrullinemic patients. Its specificity and possible clinical implications. Am J Clin Pathol. 1988 Jun;89(6):735–741. doi: 10.1093/ajcp/89.6.735. [DOI] [PubMed] [Google Scholar]