Abstract
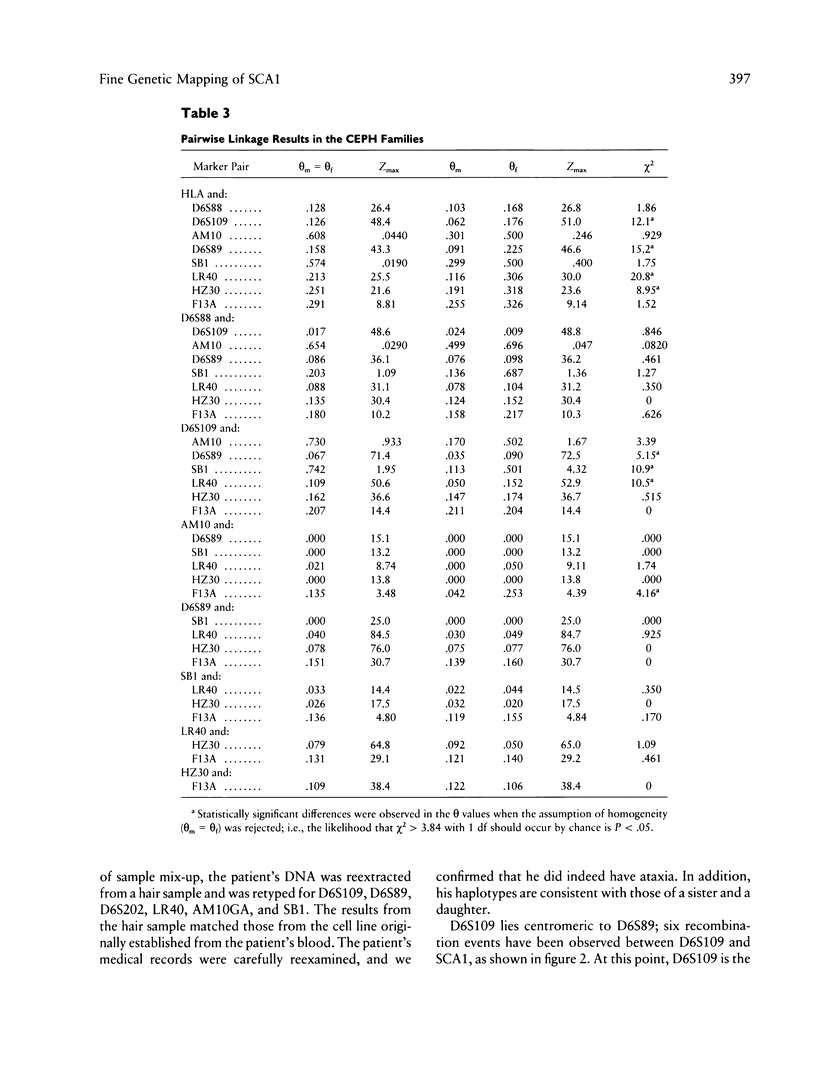
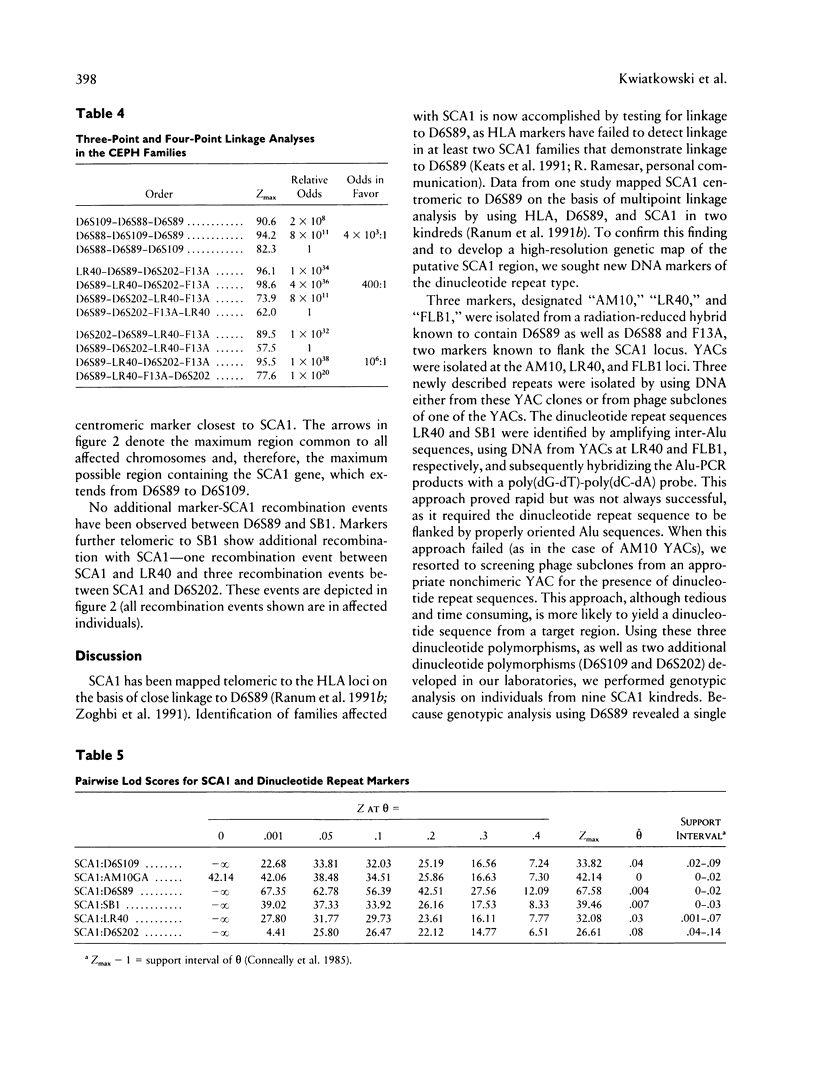
Spinocerebellar ataxia type 1 (SCA1) is an autosomal dominant disorder which is genetically linked to the short arm of chromosome 6, telomeric to the human major histocompatibility complex (HLA) and very close to D6S89. Previous multipoint linkage analysis using HLA, D6S89, and SCA1 suggested that SCA1 maps centromeric to D6S89. Data from this study using nine large kindreds indicate a maximum lod score between SCA1 and D6S89 of 67.58 at a maximum recombination fraction of .004. To localize SCA1 more precisely, we identified five dinucleotide polymorphisms near D6S89. Genotypic analyses at these polymorphic loci were carried out in nine multigeneration SCA1 kindreds and in the Centre d'Étude du Polymorphisme Humain reference families. A new marker, AM10GA, demonstrates no recombination with SCA1. The maximum lod score for AM10GA linkage to SCA1 is 42.14 at a recombination fraction of 0. Linkage analysis and analysis of recombination events confirm that SCA1 maps centromeric to D6S89 and establish the following order: CEN-D6S109–AM10GA/SCA1–D6S89–LR40–D6S202–TEL.
Full text
PDF


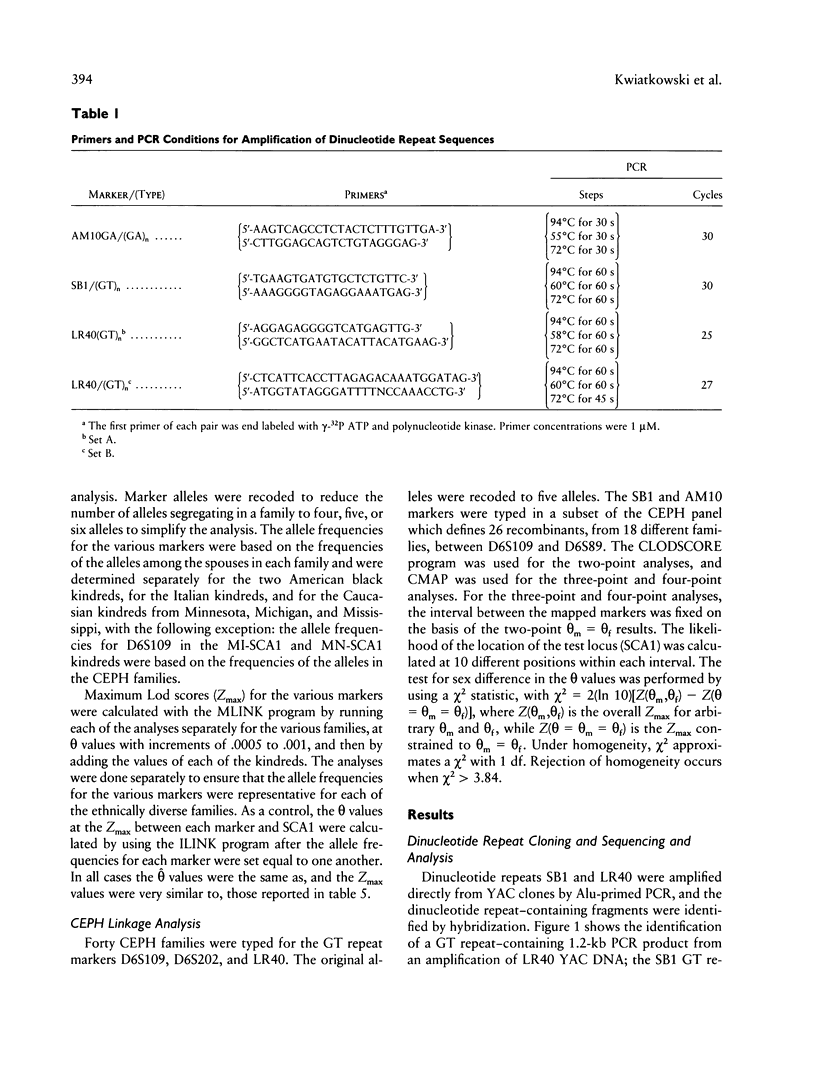
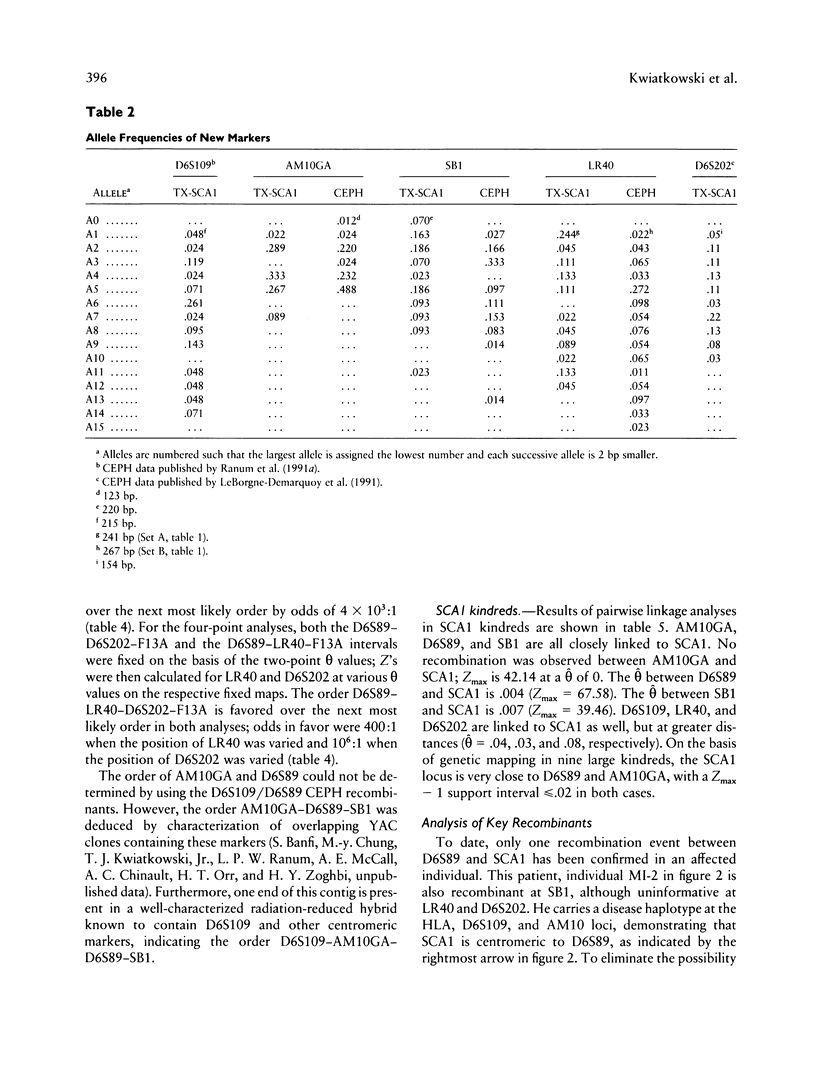


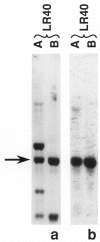
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breukel C., Wijnen J., Tops C., vd Klift H., Dauwerse H., Khan P. M. Vector-Alu PCR: a rapid step in mapping cosmids and YACs. Nucleic Acids Res. 1990 May 25;18(10):3097–3097. doi: 10.1093/nar/18.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneally P. M., Edwards J. H., Kidd K. K., Lalouel J. M., Morton N. E., Ott J., White R. Report of the Committee on Methods of Linkage Analysis and Reporting. Cytogenet Cell Genet. 1985;40(1-4):356–359. doi: 10.1159/000132186. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Boyce F. M., Kunkel L. M. Rapid detection of CA polymorphisms in cloned DNA: application to the 5' region of the dystrophin gene. Am J Hum Genet. 1991 Mar;48(3):621–627. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Haines J. L., Schut L. J., Weitkamp L. R., Thayer M., Anderson V. E. Spinocerebellar ataxia in a large kindred: age at onset, reproduction, and genetic linkage studies. Neurology. 1984 Dec;34(12):1542–1548. doi: 10.1212/wnl.34.12.1542. [DOI] [PubMed] [Google Scholar]
- Heery D. M., Gannon F., Powell R. A simple method for subcloning DNA fragments from gel slices. Trends Genet. 1990 Jun;6(6):173–173. doi: 10.1016/0168-9525(90)90158-3. [DOI] [PubMed] [Google Scholar]
- Jackson J. F., Currier R. D., Terasaki P. I., Morton N. E. Spinocerebellar ataxia and HLA linkage: risk prediction by HLA typing. N Engl J Med. 1977 May 19;296(20):1138–1141. doi: 10.1056/NEJM197705192962003. [DOI] [PubMed] [Google Scholar]
- Keats B. J., Pollack M. S., McCall A., Wilensky M. A., Ward L. J., Lu M., Zoghbi H. Y. Tight linkage of the gene for spinocerebellar ataxia to D6S89 on the short arm of chromosome 6 in a kindred for which close linkage to both HLA and F13A1 is excluded. Am J Hum Genet. 1991 Nov;49(5):972–977. [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne-Demarquoy F., Kwiatowski T. J., Jr, Zoghbi H. Y. Two dinucleotide repeat polymorphisms at the D6S202 locus. Nucleic Acids Res. 1991 Nov 11;19(21):6060–6060. doi: 10.1093/nar/19.21.6060-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nino H. E., Noreen H. J., Dubey D. P., Resch J. A., Namboodiri K., Elston R. C., Yunis E. J. A family with hereditary ataxia: HLA typing. Neurology. 1980 Jan;30(1):12–20. doi: 10.1212/wnl.30.1.12. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Chung M. Y., Duvick L. A., Zoghbi H. Y., Orr H. T. Dinucleotide repeat polymorphism at the D6S109 locus. Nucleic Acids Res. 1991 Mar 11;19(5):1171–1171. doi: 10.1093/nar/19.5.1171-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranum L. P., Duvick L. A., Rich S. S., Schut L. J., Litt M., Orr H. T. Localization of the autosomal dominant HLA-linked spinocerebellar ataxia (SCA1) locus, in two kindreds, within an 8-cM subregion of chromosome 6p. Am J Hum Genet. 1991 Jul;49(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- Spadaro M., Giunti P., Lulli P., Frontali M., Jodice C., Cappellacci S., Morellini M., Persichetti F., Trabace S., Anastasi R. HLA-linked spinocerebellar ataxia: a clinical and genetic study of large Italian kindreds. Acta Neurol Scand. 1992 Apr;85(4):257–265. doi: 10.1111/j.1600-0404.1992.tb04041.x. [DOI] [PubMed] [Google Scholar]
- Yakura H., Wakisaka A., Fujimoto S., Itakura K. Letter: Hereditary ataxia and HL-A. N Engl J Med. 1974 Jul 18;291(3):154–155. doi: 10.1056/NEJM197407182910314. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Jodice C., Sandkuijl L. A., Kwiatkowski T. J., Jr, McCall A. E., Huntoon S. A., Lulli P., Spadaro M., Litt M., Cann H. M. The gene for autosomal dominant spinocerebellar ataxia (SCA1) maps telomeric to the HLA complex and is closely linked to the D6S89 locus in three large kindreds. Am J Hum Genet. 1991 Jul;49(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Zoghbi H. Y., Pollack M. S., Lyons L. A., Ferrell R. E., Daiger S. P., Beaudet A. L. Spinocerebellar ataxia: variable age of onset and linkage to human leukocyte antigen in a large kindred. Ann Neurol. 1988 Jun;23(6):580–584. doi: 10.1002/ana.410230609. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Sandkuyl L. A., Ott J., Daiger S. P., Pollack M., O'Brien W. E., Beaudet A. L. Assignment of autosomal dominant spinocerebellar ataxia (SCA1) centromeric to the HLA region on the short arm of chromosome 6, using multilocus linkage analysis. Am J Hum Genet. 1989 Feb;44(2):255–263. [PMC free article] [PubMed] [Google Scholar]