Abstract
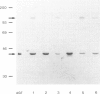
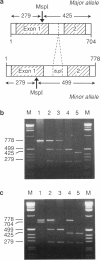
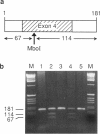
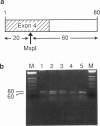
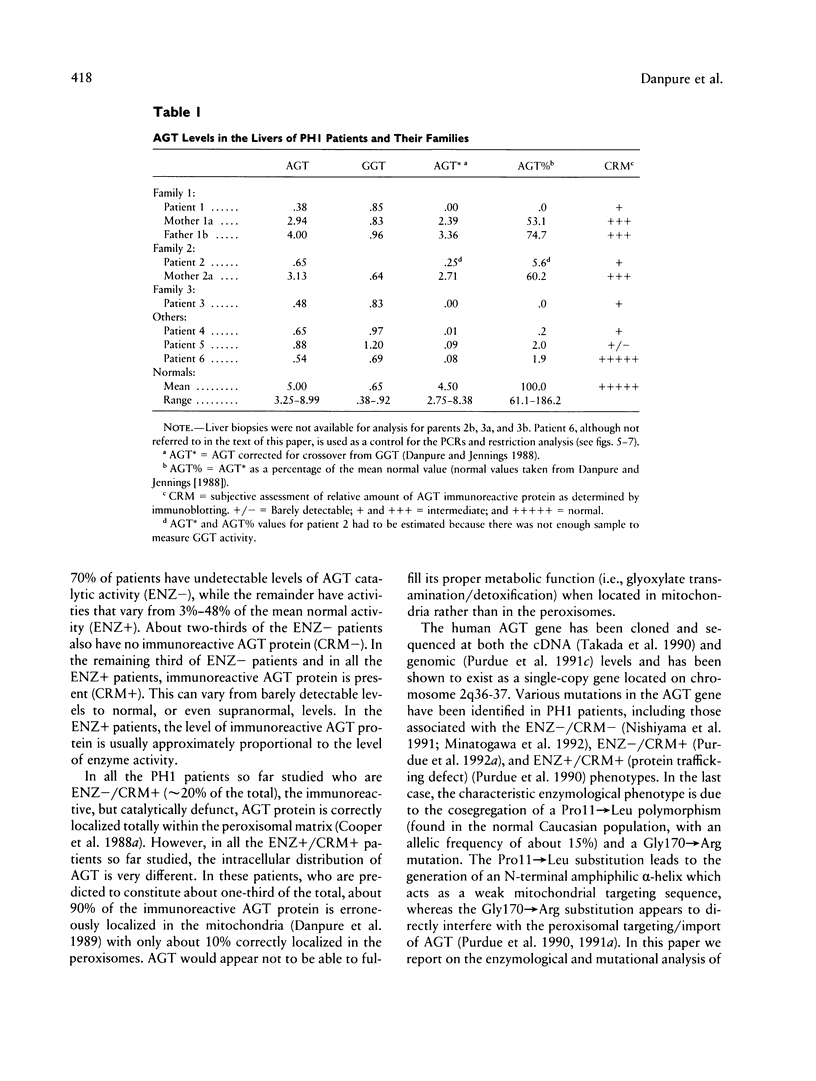
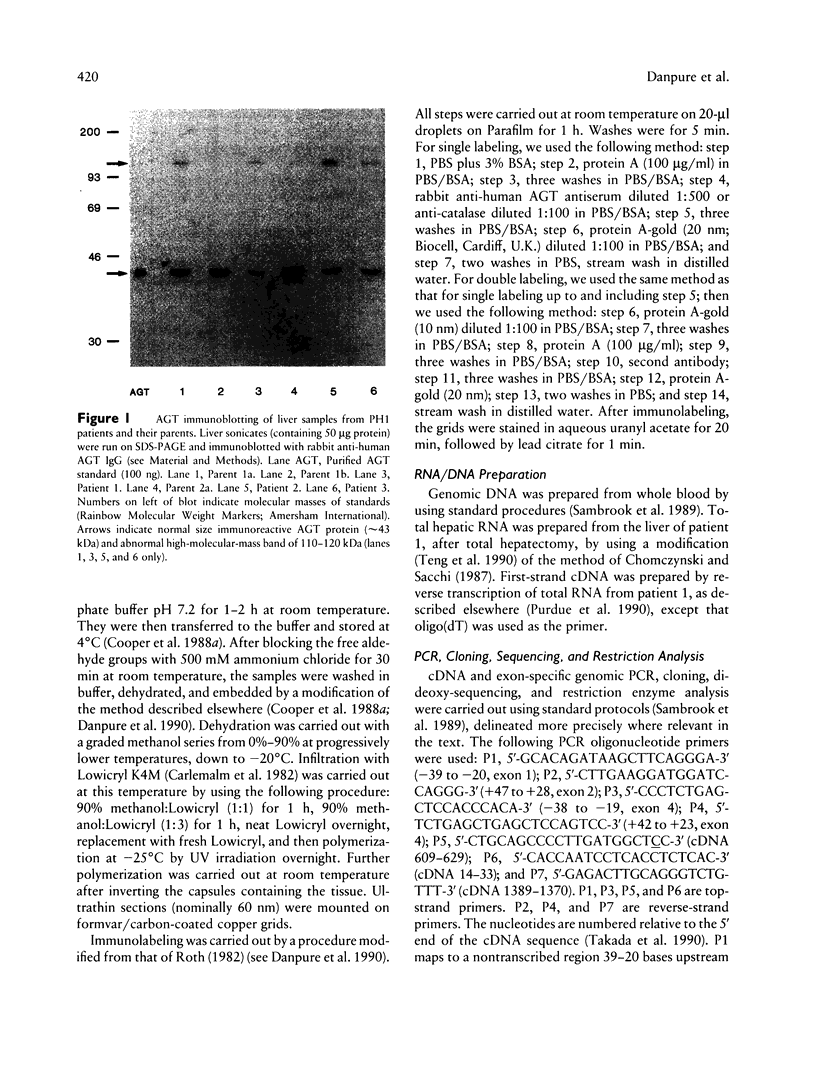
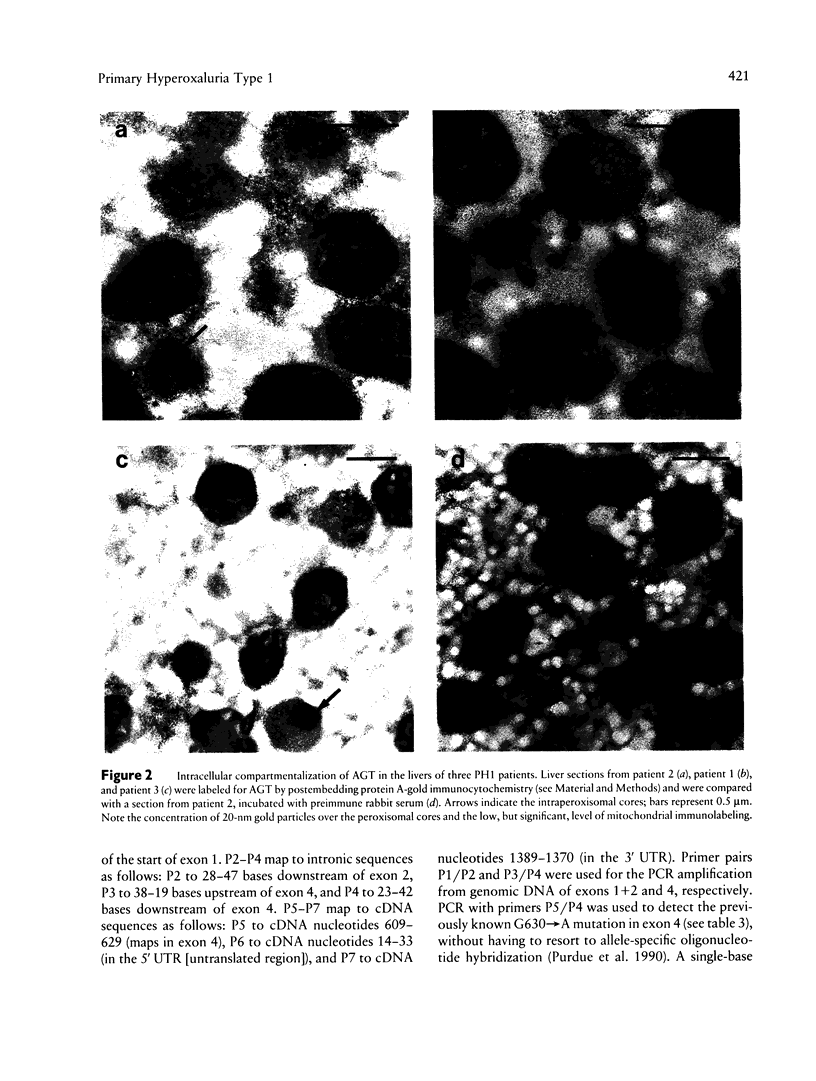
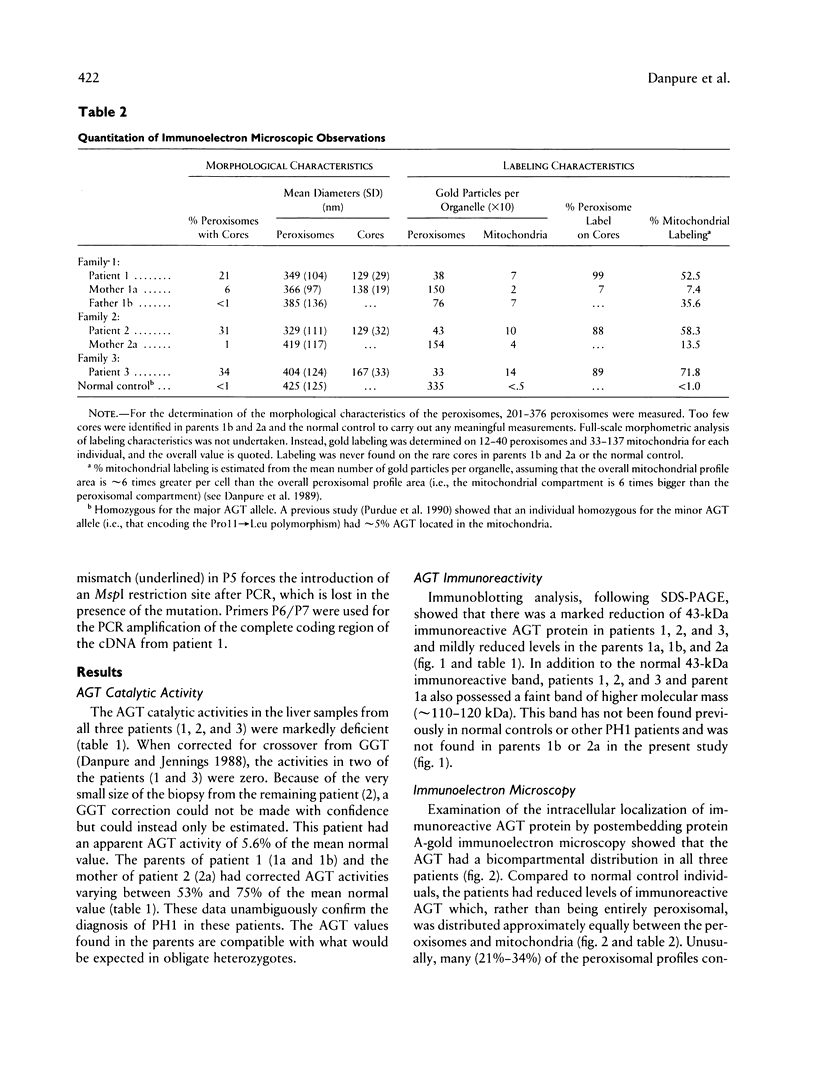
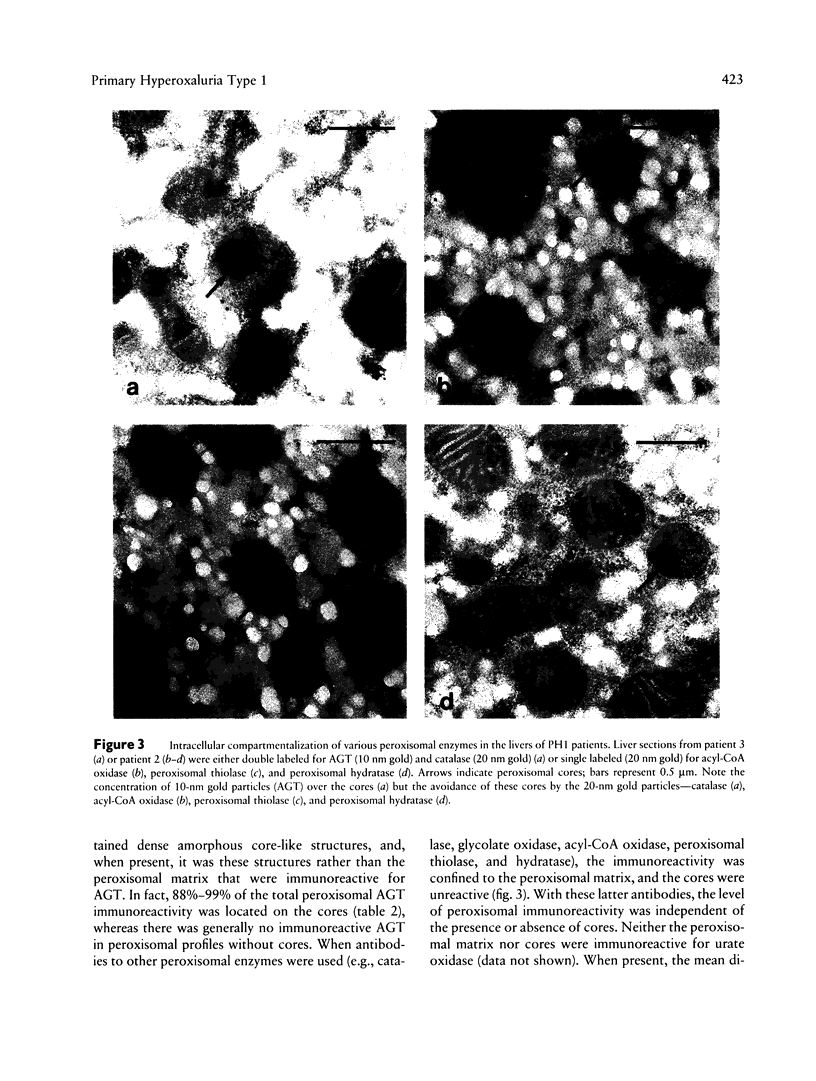
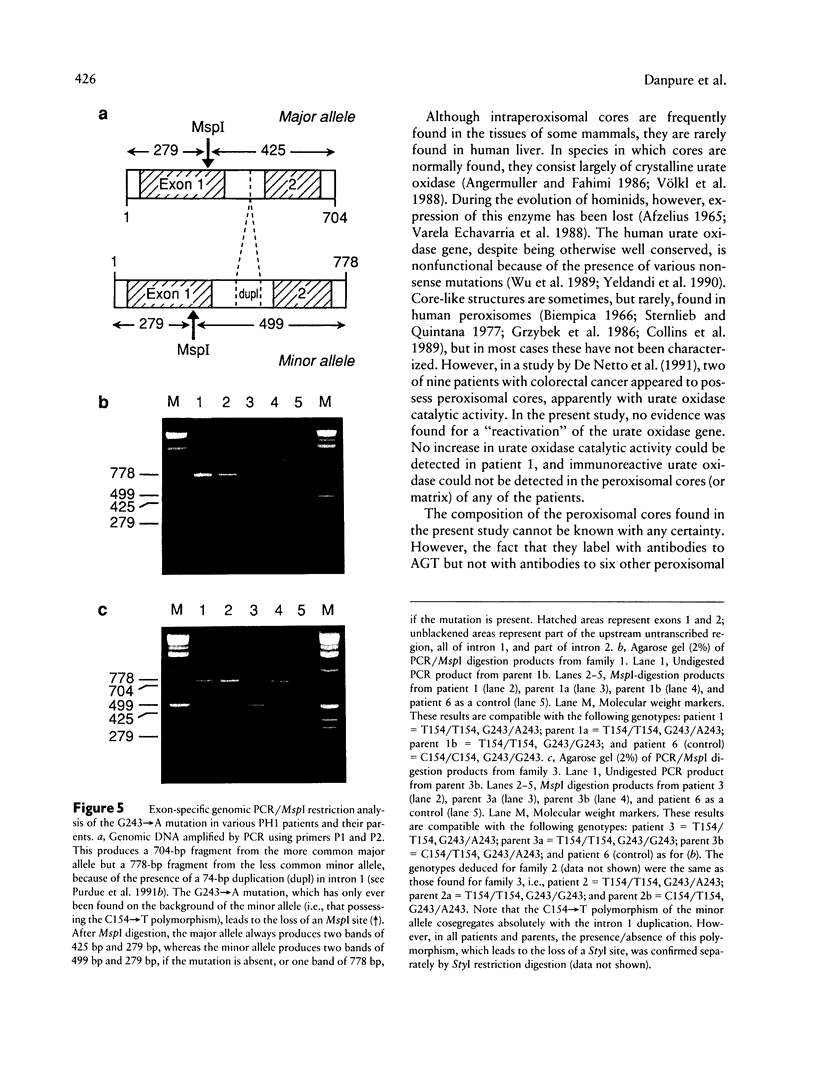
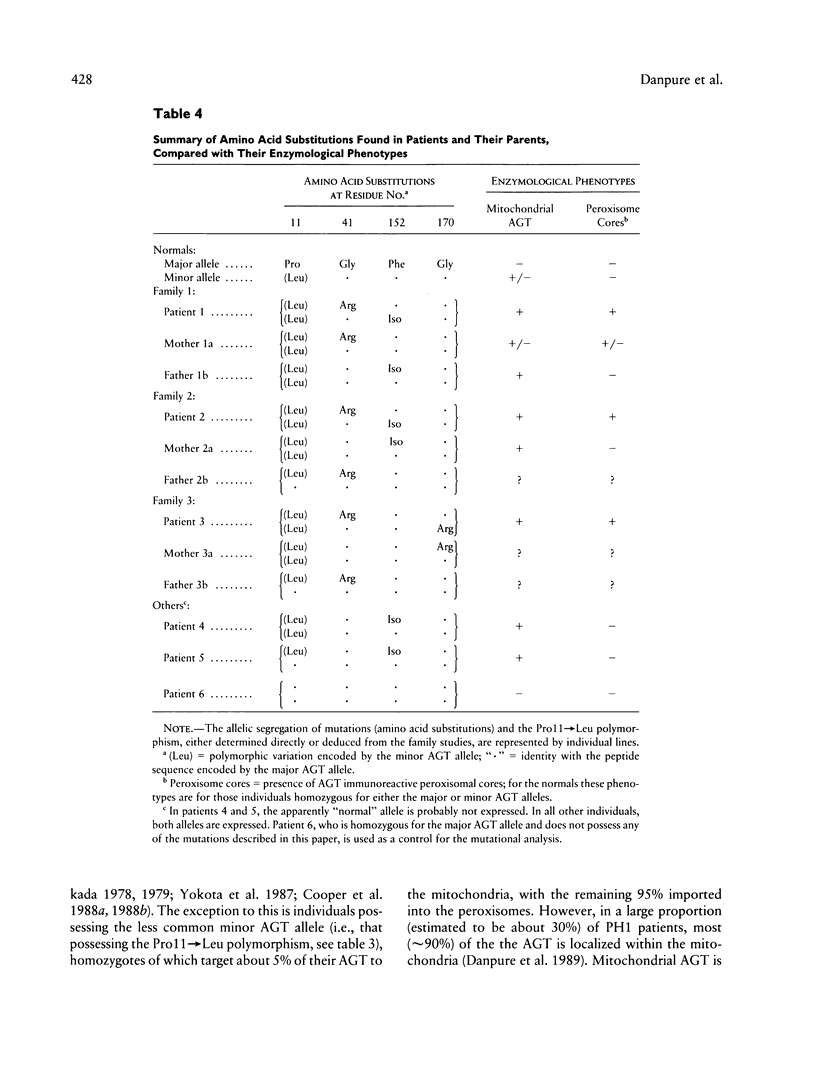
Primary hyperoxaluria type 1 (PH1) is a rare autosomal recessive disease caused by a deficiency of the liver-specific peroxisomal enzyme alanine:glyoxylate aminotransferase (AGT). Three unrelated PH1 patients, who possess a novel complex phenotype, are described. At the enzymological level, this phenotype is characterized by a complete, or nearly complete, absence of AGT catalytic activity and reduced AGT immunoreactivity. Unlike normal individuals in whom the AGT is confined to the peroxisomal matrix, the immunoreactive AGT in these three patients was distributed approximately equally between the peroxisomes and mitochondria. The peroxisomal AGT appeared to be aggregated into amorphous core-like structures in which no other peroxisomal enzymes could be identified. Mutational analysis of the AGT gene showed that two of the three patients were compound heterozygotes for two previously unrecognized point mutations which caused Gly41→Arg and Phe152→Iso amino acid substitutions. The third patient was shown to be a compound heterozygote for the Gly41→Arg mutation and a previously recognized Gly170→Arg mutation. All three patients were homozygous for the Pro11→Leu polymorphism that had been found previously with a high allelic frequency in normal populations. It is suggested that the Phe152→Iso and Gly170→Arg substitutions, which are only eighteen residues apart and located in the same highly conserved internal region of 58 amino acids, might be involved in the inhibition of peroxisomal targeting and/or import of AGT and, in combination with the Pro11→Leu polymorphism, be responsible for its aberrant mitochondrial compartmentalization. On the other hand, the Gly41→Arg substitution, either in combination with the Pro11→Leu polymorphism or by itself, is predicted to be responsible for the intraperoxisomal aggregation of the AGT protein.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop J., Jennings P. R., Danpure C. J. A new micro-assay for human liver alanine: glyoxylate aminotransferase. Clin Chim Acta. 1987 Dec;170(2-3):187–193. doi: 10.1016/0009-8981(87)90127-6. [DOI] [PubMed] [Google Scholar]
- Angermüller S., Fahimi H. D. Ultrastructural cytochemical localization of uricase in peroxisomes of rat liver. J Histochem Cytochem. 1986 Feb;34(2):159–165. doi: 10.1177/34.2.3080517. [DOI] [PubMed] [Google Scholar]
- Biempica L. Human hepatic microbodies with crystalloid cores. J Cell Biol. 1966 May;29(2):383–386. doi: 10.1083/jcb.29.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Collins J. C., Scheinberg I. H., Giblin D. R., Sternlieb I. Hepatic peroxisomal abnormalities in abetalipoproteinemia. Gastroenterology. 1989 Sep;97(3):766–770. doi: 10.1016/0016-5085(89)90651-3. [DOI] [PubMed] [Google Scholar]
- Cooper P. J., Danpure C. J., Wise P. J., Guttridge K. M. Immunocytochemical localization of human hepatic alanine: glyoxylate aminotransferase in control subjects and patients with primary hyperoxaluria type 1. J Histochem Cytochem. 1988 Oct;36(10):1285–1294. doi: 10.1177/36.10.3418107. [DOI] [PubMed] [Google Scholar]
- Danpure C. J., Cooper P. J., Wise P. J., Jennings P. R. An enzyme trafficking defect in two patients with primary hyperoxaluria type 1: peroxisomal alanine/glyoxylate aminotransferase rerouted to mitochondria. J Cell Biol. 1989 Apr;108(4):1345–1352. doi: 10.1083/jcb.108.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danpure C. J., Guttridge K. M., Fryer P., Jennings P. R., Allsop J., Purdue P. E. Subcellular distribution of hepatic alanine:glyoxylate aminotransferase in various mammalian species. J Cell Sci. 1990 Dec;97(Pt 4):669–678. doi: 10.1242/jcs.97.4.669. [DOI] [PubMed] [Google Scholar]
- Danpure C. J., Jennings P. R. Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I. FEBS Lett. 1986 May 26;201(1):20–24. doi: 10.1016/0014-5793(86)80563-4. [DOI] [PubMed] [Google Scholar]
- Danpure C. J. Molecular and clinical heterogeneity in primary hyperoxaluria type 1. Am J Kidney Dis. 1991 Apr;17(4):366–369. doi: 10.1016/s0272-6386(12)80624-x. [DOI] [PubMed] [Google Scholar]
- De-Netto L. A., Tappia P. S., Malik Z. A., Wood A. J., Mann V. M., Jones C. J., Burdett K., Neoptolemos J. P., Connock M. J. Human hepatic peroxisomes with crystalloid cores associated with urate oxidase activity. Adv Exp Med Biol. 1991;309A:373–376. doi: 10.1007/978-1-4899-2638-8_85. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R., Ledley F. D. Disruption of phase during PCR amplification and cloning of heterozygous target sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5153–5156. doi: 10.1093/nar/18.17.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minatogawa Y., Tone S., Allsop J., Purdue P. E., Takada Y., Danpur C. J., Kido R. A serine-to-phenylalanine substitution leads to loss of alanine:glyoxylate aminotransferase catalytic activity and immunoreactivity in a patient with primary hyperoxaluria type 1. Hum Mol Genet. 1992 Nov;1(8):643–644. doi: 10.1093/hmg/1.8.643. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Funai T., Katafuchi R., Hattori F., Onoyama K., Ichiyama A. Primary hyperoxaluria type I due to a point mutation of T to C in the coding region of the serine:pyruvate aminotransferase gene. Biochem Biophys Res Commun. 1991 May 15;176(3):1093–1099. doi: 10.1016/0006-291x(91)90396-o. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Takada Y. Peroxisomal localization of serine:pyruvate aminotransferase in human liver. J Biol Chem. 1978 Nov 10;253(21):7598–7600. [PubMed] [Google Scholar]
- Oda T., Miyajima H., Suzuki Y., Ichiyama A. Nucleotide sequence of the cDNA encoding the precursor for mitochondrial serine:pyruvate aminotransferase of rat liver. Eur J Biochem. 1987 Nov 2;168(3):537–542. doi: 10.1111/j.1432-1033.1987.tb13451.x. [DOI] [PubMed] [Google Scholar]
- Purdue P. E., Allsop J., Isaya G., Rosenberg L. E., Danpure C. J. Mistargeting of peroxisomal L-alanine:glyoxylate aminotransferase to mitochondria in primary hyperoxaluria patients depends upon activation of a cryptic mitochondrial targeting sequence by a point mutation. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10900–10904. doi: 10.1073/pnas.88.23.10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue P. E., Lumb M. J., Allsop J., Danpure C. J. An intronic duplication in the alanine: glyoxylate aminotransferase gene facilitates identification of mutations in compound heterozygote patients with primary hyperoxaluria type 1. Hum Genet. 1991 Aug;87(4):394–396. doi: 10.1007/BF00197154. [DOI] [PubMed] [Google Scholar]
- Purdue P. E., Lumb M. J., Danpure C. J. Molecular evolution of alanine/glyoxylate aminotransferase 1 intracellular targeting. Analysis of the marmoset and rabbit genes. Eur J Biochem. 1992 Jul 15;207(2):757–766. doi: 10.1111/j.1432-1033.1992.tb17106.x. [DOI] [PubMed] [Google Scholar]
- Purdue P. E., Lumb M. J., Fox M., Griffo G., Hamon-Benais C., Povey S., Danpure C. J. Characterization and chromosomal mapping of a genomic clone encoding human alanine:glyoxylate aminotransferase. Genomics. 1991 May;10(1):34–42. doi: 10.1016/0888-7543(91)90481-s. [DOI] [PubMed] [Google Scholar]
- Purdue P. E., Takada Y., Danpure C. J. Identification of mutations associated with peroxisome-to-mitochondrion mistargeting of alanine/glyoxylate aminotransferase in primary hyperoxaluria type 1. J Cell Biol. 1990 Dec;111(6 Pt 1):2341–2351. doi: 10.1083/jcb.111.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlieb I., Quintana N. The peroxisomes of human hepatocytes. Lab Invest. 1977 Feb;36(2):140–149. [PubMed] [Google Scholar]
- Takada Y., Kaneko N., Esumi H., Purdue P. E., Danpure C. J. Human peroxisomal L-alanine: glyoxylate aminotransferase. Evolutionary loss of a mitochondrial targeting signal by point mutation of the initiation codon. Biochem J. 1990 Jun 1;268(2):517–520. doi: 10.1042/bj2680517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. Subcellular distribution, and physical and immunological properties of hepatic alanine: glyoxylate aminotransferase isoenzymes in different mammalian species. Comp Biochem Physiol B. 1982;72(4):597–604. doi: 10.1016/0305-0491(82)90512-0. [DOI] [PubMed] [Google Scholar]
- Teng B., Verp M., Salomon J., Davidson N. O. Apolipoprotein B messenger RNA editing is developmentally regulated and widely expressed in human tissues. J Biol Chem. 1990 Nov 25;265(33):20616–20620. [PubMed] [Google Scholar]
- Thompson J. S., Richardson K. E. Isolation and characterization of an L-alanine: glyoxylate aminotransferase from human liver. J Biol Chem. 1967 Aug 25;242(16):3614–3619. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Echavarría A., Montes de Oca-Luna R., Barrera-Saldaña H. A. Uricase protein sequences: conserved during vertebrate evolution but absent in humans. FASEB J. 1988 Dec;2(15):3092–3096. doi: 10.1096/fasebj.2.15.3192041. [DOI] [PubMed] [Google Scholar]
- Völkl A., Baumgart E., Fahimi H. D. Localization of urate oxidase in the crystalline cores of rat liver peroxisomes by immunocytochemistry and immunoblotting. J Histochem Cytochem. 1988 Apr;36(4):329–336. doi: 10.1177/36.4.3346536. [DOI] [PubMed] [Google Scholar]
- Watts R. W., Danpure C. J., De Pauw L., Toussaint Combined liver-kidney and isolated liver transplantations for primary hyperoxaluria type 1: the European experience. The European Study Group on Transplantation in Hyperoxaluria Type 1. Nephrol Dial Transplant. 1991;6(7):502–511. doi: 10.1093/ndt/6.7.502. [DOI] [PubMed] [Google Scholar]
- Wise P. J., Danpure C. J., Jennings P. R. Immunological heterogeneity of hepatic alanine:glyoxylate aminotransferase in primary hyperoxaluria type 1. FEBS Lett. 1987 Sep 28;222(1):17–20. doi: 10.1016/0014-5793(87)80183-7. [DOI] [PubMed] [Google Scholar]
- Wu X. W., Lee C. C., Muzny D. M., Caskey C. T. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9412–9416. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeldandi A. V., Wang X. D., Alvares K., Kumar S., Rao M. S., Reddy J. K. Human urate oxidase gene: cloning and partial sequence analysis reveal a stop codon within the fifth exon. Biochem Biophys Res Commun. 1990 Sep 14;171(2):641–646. doi: 10.1016/0006-291x(90)91194-w. [DOI] [PubMed] [Google Scholar]
- Yokota S., Oda T., Ichiyama A. Immunocytochemical localization of serine: pyruvate aminotransferase in peroxisomes of the human liver parenchymal cells. Histochemistry. 1987;87(6):601–606. doi: 10.1007/BF00492477. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]