Abstract
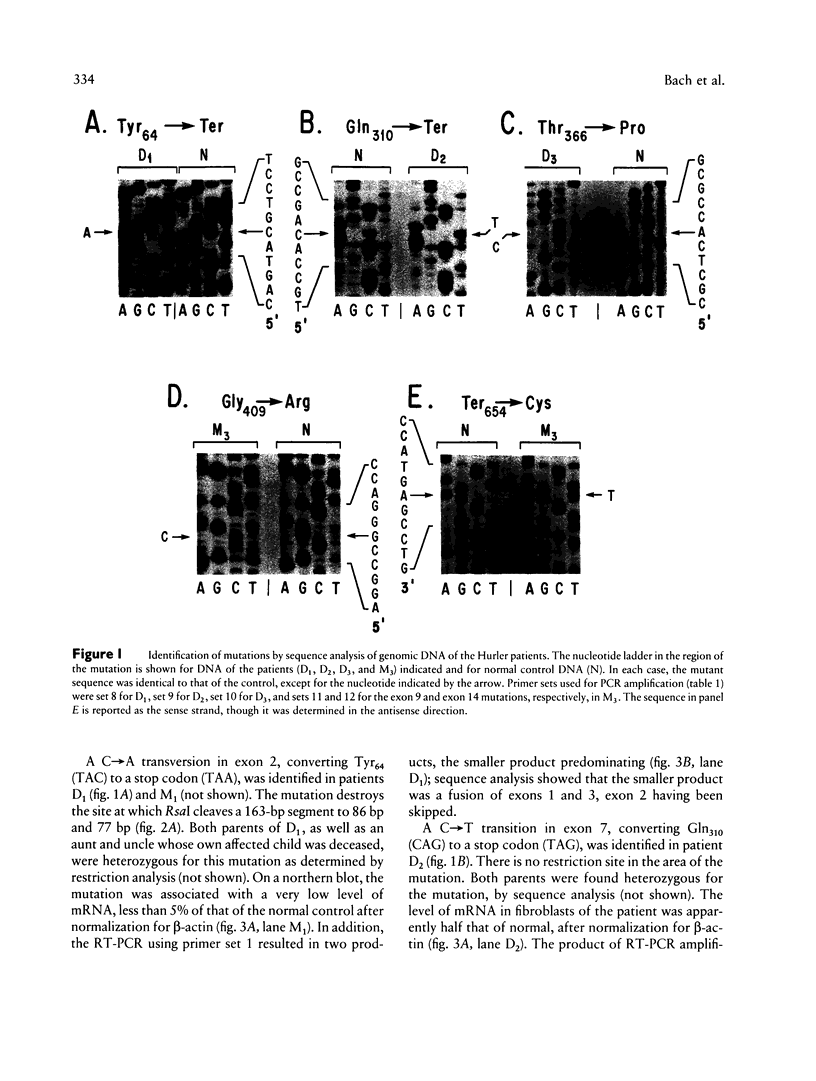
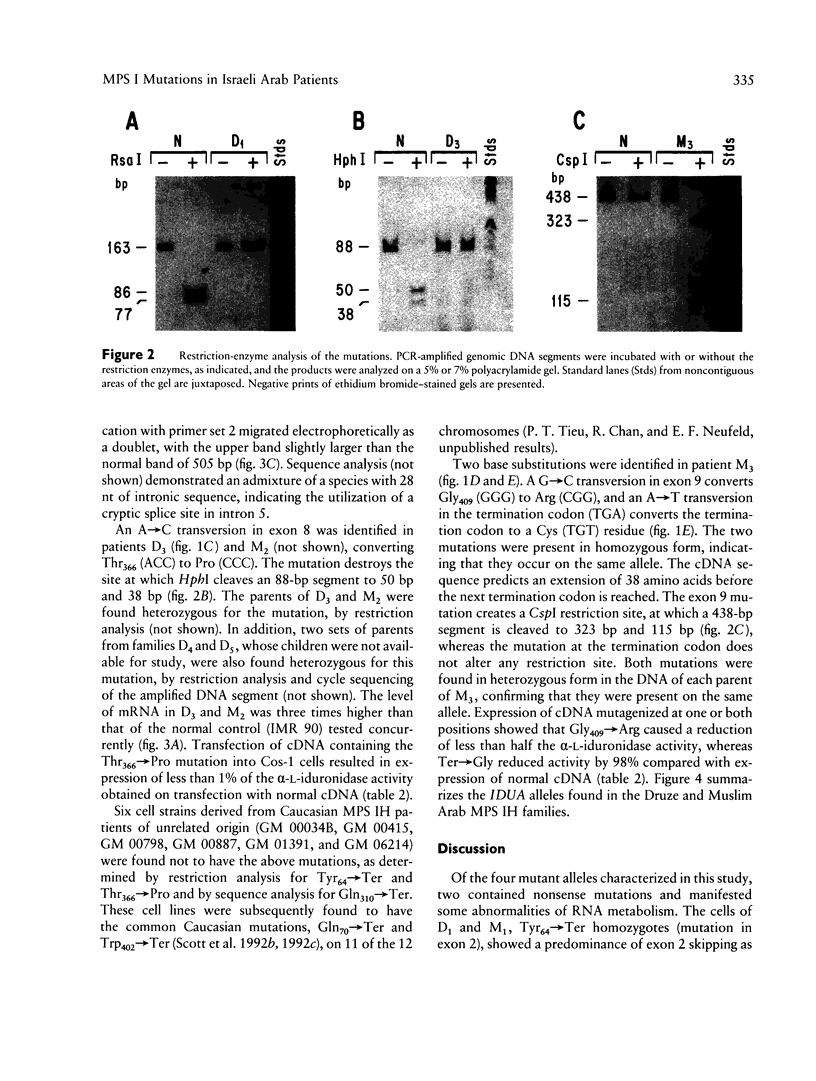
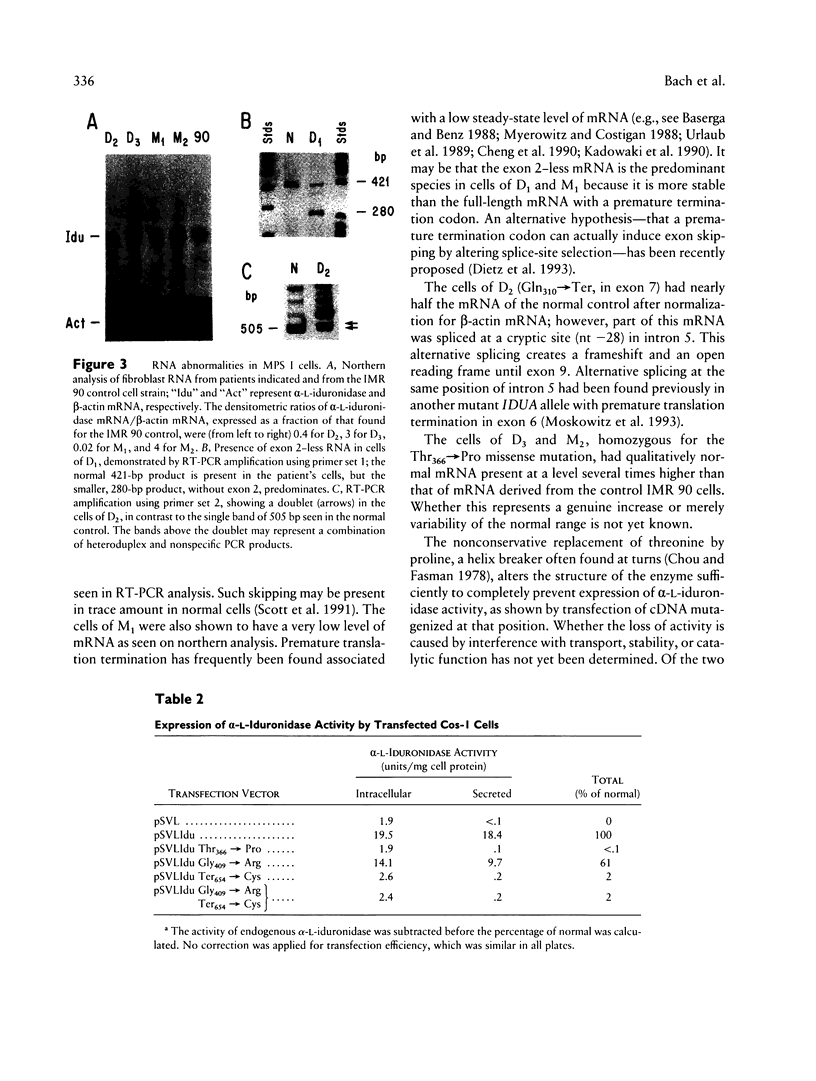
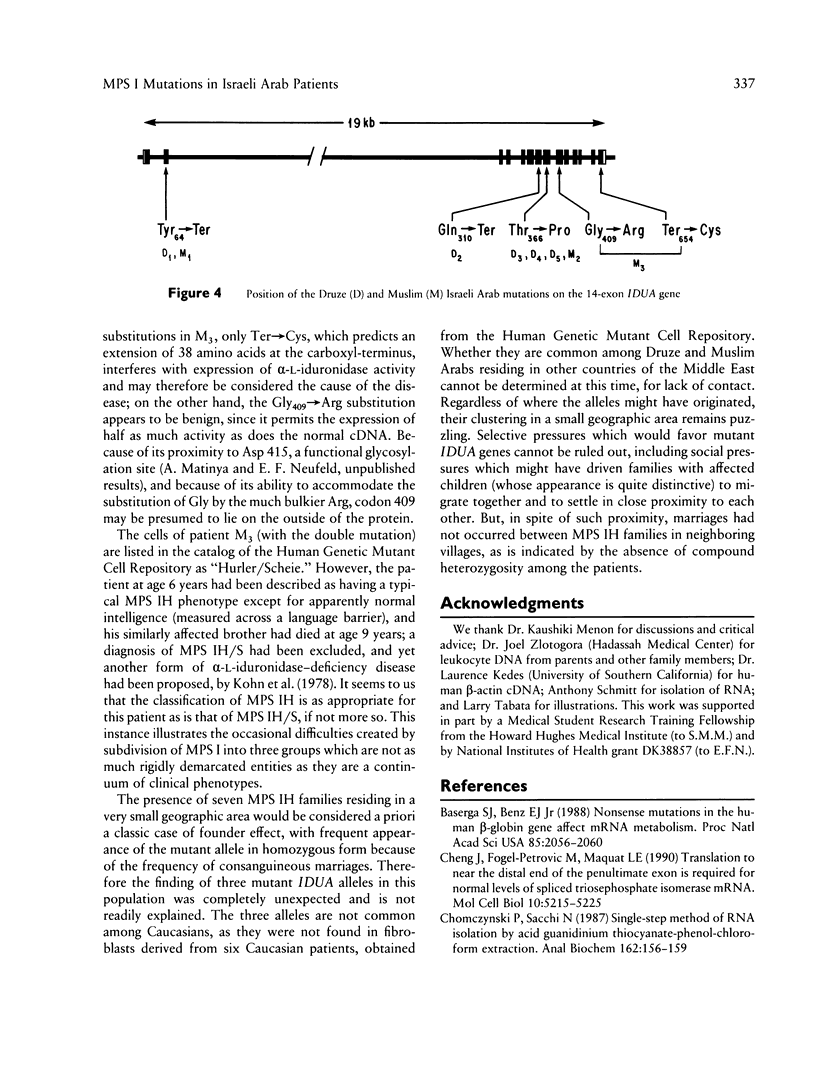
The mutations underlying Hurler syndrome (mucopolysaccharidosis IH) in Druze and Muslim Israeli Arab patients have been characterized. Four alleles were identified, using a combination of (a) PCR amplification of reverse-transcribed RNA or genomic DNA segments, (b) cycle sequencing of PCR products, and (c) restriction-enzyme analysis. One allele has two amino acid substitutions, Gly409-->Arg in exon 9 and Ter-->Cys in exon 14. The other three alleles have mutations in exon 2 (Tyr64-->Ter), exon 7 (Gln310-->Ter), or exon 8 (Thr366-->Pro). Transfection of mutagenized cDNAs into Cos-1 cells showed that two missense mutations, Thr366-->Pro and Ter-->Cys, permitted the expression of only trace amounts of alpha-L-iduronidase activity, whereas Gly409-->Arg permitted the expression of 60% as much enzyme as did the normal cDNA. The nonsense mutations were associated with abnormalities of RNA processing: (1) both a very low level of mRNA and skipping of exon 2 for Tyr64-->Ter and (2) utilization of a cryptic splice site for Gln310-->Ter. In all instances, the probands were found homozygous, and the parents heterozygous, for the mutant alleles, as anticipated from the consanguinity in each family. The two-mutation allele was identified in a family from Gaza; the other three alleles were found in seven families, five of them Druze, residing in a very small area of northern Israel. Since such clustering suggests a classic founder effect, the presence of three mutant alleles of the IDUA gene was unexpected.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Fogel-Petrovic M., Maquat L. E. Translation to near the distal end of the penultimate exon is required for normal levels of spliced triosephosphate isomerase mRNA. Mol Cell Biol. 1990 Oct;10(10):5215–5225. doi: 10.1128/mcb.10.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz H. C., Valle D., Francomano C. A., Kendzior R. J., Jr, Pyeritz R. E., Cutting G. R. The skipping of constitutive exons in vivo induced by nonsense mutations. Science. 1993 Jan 29;259(5095):680–683. doi: 10.1126/science.8430317. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn G., Bach G., Lasch E., Massri M. E., Legum C., Cohen M. M. A new phenotypic variant of alpha-L-iduronidase deficiency. Monogr Hum Genet. 1978;10:7–10. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lowry R. B., Renwick D. H. Relative frequency of the Hurler and Hunter syndromes. N Engl J Med. 1971 Jan 28;284(4):221–222. doi: 10.1056/NEJM197101282840425. [DOI] [PubMed] [Google Scholar]
- Menon K. P., Tieu P. T., Neufeld E. F. Architecture of the canine IDUA gene and mutation underlying canine mucopolysaccharidosis I. Genomics. 1992 Nov;14(3):763–768. doi: 10.1016/s0888-7543(05)80182-x. [DOI] [PubMed] [Google Scholar]
- Moskowitz S. M., Tieu P. T., Neufeld E. F. A deletion/insertion mutation in the IDUA gene in a Libyan Jewish patient with Hurler syndrome (mucopolysaccharidosis IH). Hum Mutat. 1993;2(1):71–73. doi: 10.1002/humu.1380020113. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Costigan F. C. The major defect in Ashkenazi Jews with Tay-Sachs disease is an insertion in the gene for the alpha-chain of beta-hexosaminidase. J Biol Chem. 1988 Dec 15;263(35):18587–18589. [PubMed] [Google Scholar]
- Paw B. H., Tieu P. T., Kaback M. M., Lim J., Neufeld E. F. Frequency of three Hex A mutant alleles among Jewish and non-Jewish carriers identified in a Tay-Sachs screening program. Am J Hum Genet. 1990 Oct;47(4):698–705. [PMC free article] [PubMed] [Google Scholar]
- Paw B. H., Wood L. C., Neufeld E. F. A third mutation at the CpG dinucleotide of codon 504 and a silent mutation at codon 506 of the HEX A gene. Am J Hum Genet. 1991 Jun;48(6):1139–1146. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap T., Bach G. Incidence of mucopolysaccharidoses in Israel: is Hunter disease a "Jewish disease"? Hum Genet. 1980;56(2):221–223. doi: 10.1007/BF00295699. [DOI] [PubMed] [Google Scholar]
- Scott H. S., Anson D. S., Orsborn A. M., Nelson P. V., Clements P. R., Morris C. P., Hopwood J. J. Human alpha-L-iduronidase: cDNA isolation and expression. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9695–9699. doi: 10.1073/pnas.88.21.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. S., Guo X. H., Hopwood J. J., Morris C. P. Structure and sequence of the human alpha-L-iduronidase gene. Genomics. 1992 Aug;13(4):1311–1313. doi: 10.1016/0888-7543(92)90053-u. [DOI] [PubMed] [Google Scholar]
- Scott H. S., Litjens T., Hopwood J. J., Morris C. P. A common mutation for mucopolysaccharidosis type I associated with a severe Hurler syndrome phenotype. Hum Mutat. 1992;1(2):103–108. doi: 10.1002/humu.1380010204. [DOI] [PubMed] [Google Scholar]
- Scott H. S., Litjens T., Nelson P. V., Brooks D. A., Hopwood J. J., Morris C. P. alpha-L-iduronidase mutations (Q70X and P533R) associate with a severe Hurler phenotype. Hum Mutat. 1992;1(4):333–339. doi: 10.1002/humu.1380010412. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Johnstone E. M., Little S. P., Hsiung H. M. Direct DNA sequencing of cDNA inserts from plaques using the linear polymerase chain reaction. Biotechniques. 1990 Jul;9(1):48–passim. [PubMed] [Google Scholar]
- Stoltzfus L. J., Sosa-Pineda B., Moskowitz S. M., Menon K. P., Dlott B., Hooper L., Teplow D. B., Shull R. M., Neufeld E. F. Cloning and characterization of cDNA encoding canine alpha-L-iduronidase. mRNA deficiency in mucopolysaccharidosis I dog. J Biol Chem. 1992 Apr 5;267(10):6570–6575. [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]