Abstract
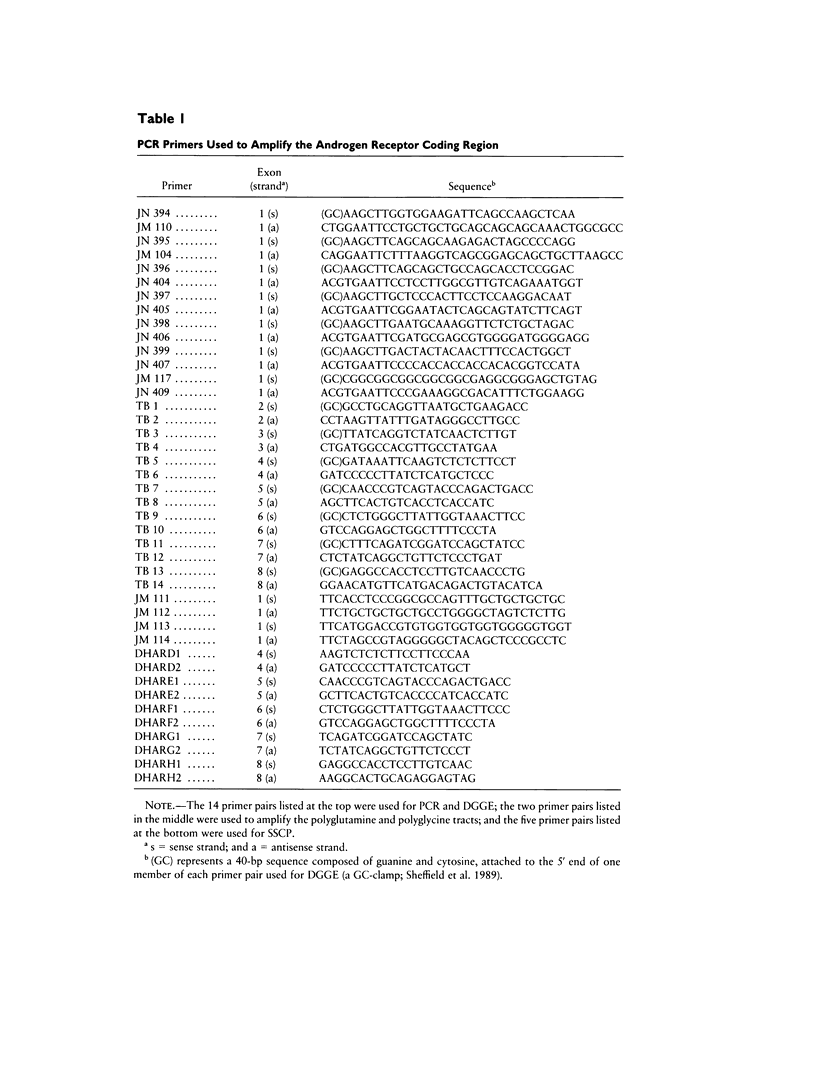
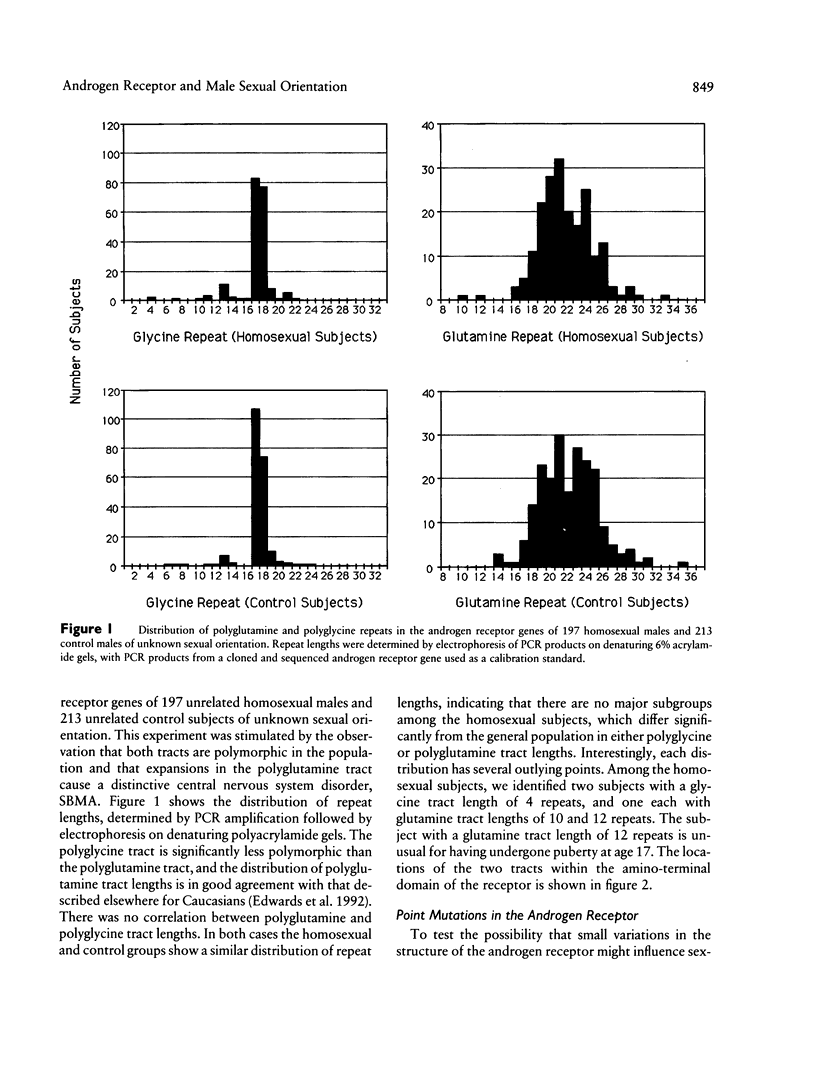
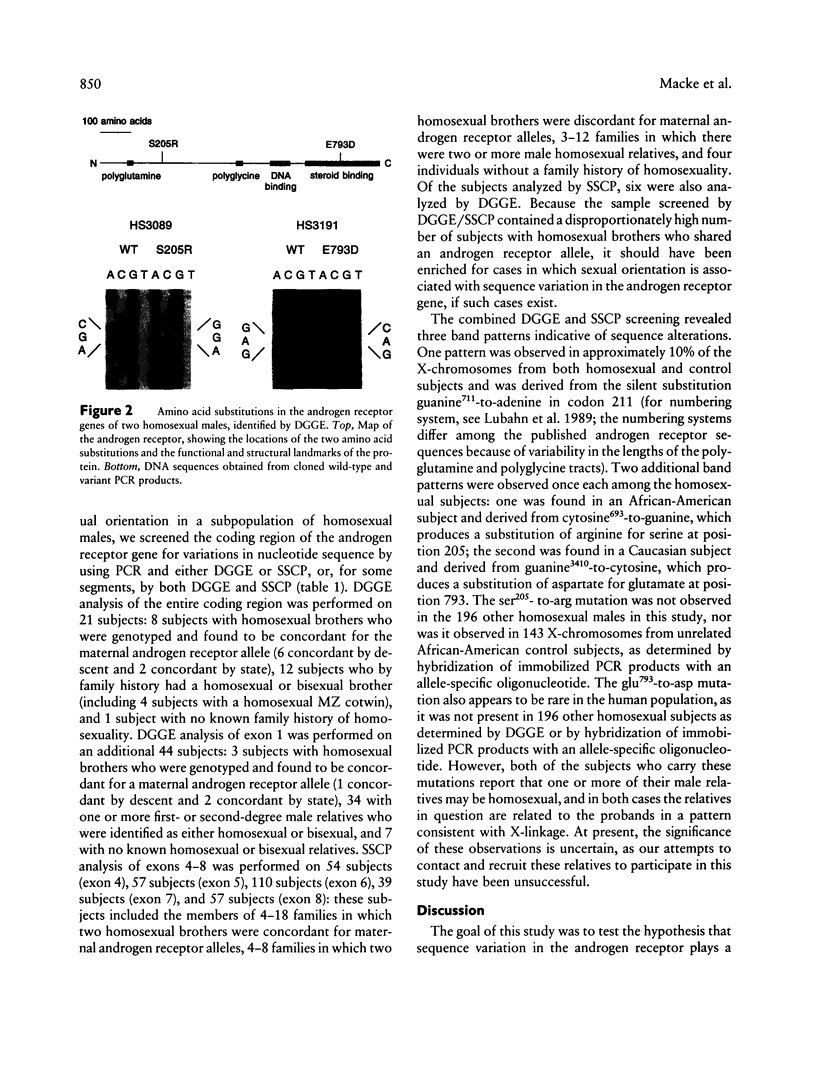
To test the hypothesis that DNA sequence variation in the androgen receptor gene plays a causal role in the development of male sexual orientation, we have (1) measured the degree of concordance of androgen receptor alleles in 36 pairs of homosexual brothers, (2) compared the lengths of polyglutamine and polyglycine tracts in the amino-terminal domain of the androgen receptor in a sample of 197 homosexual males and 213 unselected subjects, and (3) screened the the entire androgen receptor coding region for sequence variation by PCR and denaturing gradient-gel electrophoresis (DGGE) and/or single-strand conformation polymorphism analysis in 20 homosexual males with homosexual or bisexual brothers and one homosexual male with no homosexual brothers, and screened the amino-terminal domain of the receptor for sequence variation in an additional 44 homosexual males, 37 of whom had one or more first- or second-degree male relatives who were either homosexual or bisexual. These analyses show that (1) homosexual brothers are as likely to be discordant as concordant for androgen receptor alleles; (2) there are no large-scale differences between the distributions of polyglycine or polyglutamine tract lengths in the homosexual and control groups; and (3) coding region sequence variation is not commonly found within the androgen receptor gene of homosexual men. The DGGE screen identified two rare amino acid substitutions, ser205-to-arg and glu793-to-asp, the biological significance of which is unknown.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. S., Gorski R. A. Sexual orientation and the size of the anterior commissure in the human brain. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7199–7202. doi: 10.1073/pnas.89.15.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Pillard R. C. A genetic study of male sexual orientation. Arch Gen Psychiatry. 1991 Dec;48(12):1089–1096. doi: 10.1001/archpsyc.1991.01810360053008. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Pillard R. C., Neale M. C., Agyei Y. Heritable factors influence sexual orientation in women. Arch Gen Psychiatry. 1993 Mar;50(3):217–223. doi: 10.1001/archpsyc.1993.01820150067007. [DOI] [PubMed] [Google Scholar]
- Batch J. A., Williams D. M., Davies H. R., Brown B. D., Evans B. A., Hughes I. A., Patterson M. N. Androgen receptor gene mutations identified by SSCP in fourteen subjects with androgen insensitivity syndrome. Hum Mol Genet. 1992 Oct;1(7):497–503. doi: 10.1093/hmg/1.7.497. [DOI] [PubMed] [Google Scholar]
- Dörner G., Rohde W., Stahl F., Krell L., Masius W. G. A neuroendocrine predisposition for homosexuality in men. Arch Sex Behav. 1975 Jan;4(1):1–8. doi: 10.1007/BF01541882. [DOI] [PubMed] [Google Scholar]
- Dörner G. Sexual differentiation of the brain. Vitam Horm. 1980;38:325–381. doi: 10.1016/s0083-6729(08)60488-4. [DOI] [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Ehrhardt A. A., Meyer-Bahlburg H. F. Effects of prenatal sex hormones on gender-related behavior. Science. 1981 Mar 20;211(4488):1312–1318. doi: 10.1126/science.7209510. [DOI] [PubMed] [Google Scholar]
- Green R. Gender identity in childhood and later sexual orientation: follow-up of 78 males. Am J Psychiatry. 1985 Mar;142(3):339–341. doi: 10.1176/ajp.142.3.339. [DOI] [PubMed] [Google Scholar]
- Jenster G., van der Korput J. A., Trapman J., Brinkmann A. O. Functional domains of the human androgen receptor. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):671–675. doi: 10.1016/0960-0760(92)90402-5. [DOI] [PubMed] [Google Scholar]
- KALLMANN F. J. Comparative twin study on the genetic aspects of male homosexuality. J Nerv Ment Dis. 1952 Apr;115(4):283–297. [PubMed] [Google Scholar]
- King M., McDonald E. Homosexuals who are twins. A study of 46 probands. Br J Psychiatry. 1992 Mar;160:407–409. doi: 10.1192/bjp.160.3.407. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Roling D. B., Harding A. E., Warner C. L., Spiegel R., Hausmanowa-Petrusewicz I., Yee W. C., Fischbeck K. H. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992 Dec;2(4):301–304. doi: 10.1038/ng1292-301. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991 Aug 30;253(5023):1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Brown T. R., Simental J. A., Higgs H. N., Migeon C. J., Wilson E. M., French F. S. Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9534–9538. doi: 10.1073/pnas.86.23.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaul M. J., Marcelli M., Zoppi S., Griffin J. E., Wilson J. D. Genetic basis of endocrine disease. 4. The spectrum of mutations in the androgen receptor gene that causes androgen resistance. J Clin Endocrinol Metab. 1993 Jan;76(1):17–23. doi: 10.1210/jcem.76.1.8421085. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg H. F. Psychoendocrine research on sexual orientation. Current status and future options. Prog Brain Res. 1984;61:375–398. doi: 10.1016/S0079-6123(08)64448-9. [DOI] [PubMed] [Google Scholar]
- Money J., Schwartz M., Lewis V. G. Adult erotosexual status and fetal hormonal masculinization and demasculinization: 46,XX congenital virilizing adrenal hyperplasia and 46,XY androgen-insensitivity syndrome compared. Psychoneuroendocrinology. 1984;9(4):405–414. doi: 10.1016/0306-4530(84)90048-9. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Pillard R. C., Weinrich J. D. Evidence of familial nature of male homosexuality. Arch Gen Psychiatry. 1986 Aug;43(8):808–812. doi: 10.1001/archpsyc.1986.01800080094012. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab D. F., Hofman M. A. An enlarged suprachiasmatic nucleus in homosexual men. Brain Res. 1990 Dec 24;537(1-2):141–148. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- Zuger B. Early effeminate behavior in boys. Outcome and significance for homosexuality. J Nerv Ment Dis. 1984 Feb;172(2):90–97. doi: 10.1097/00005053-198402000-00005. [DOI] [PubMed] [Google Scholar]