Abstract
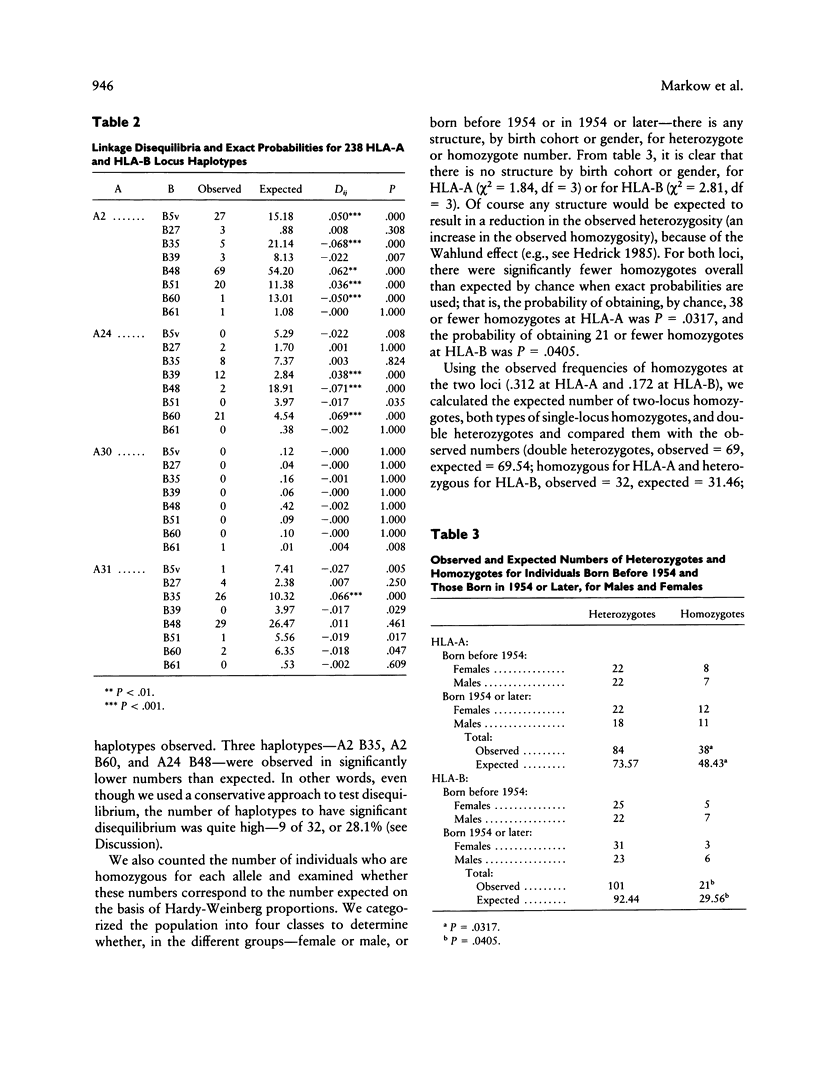
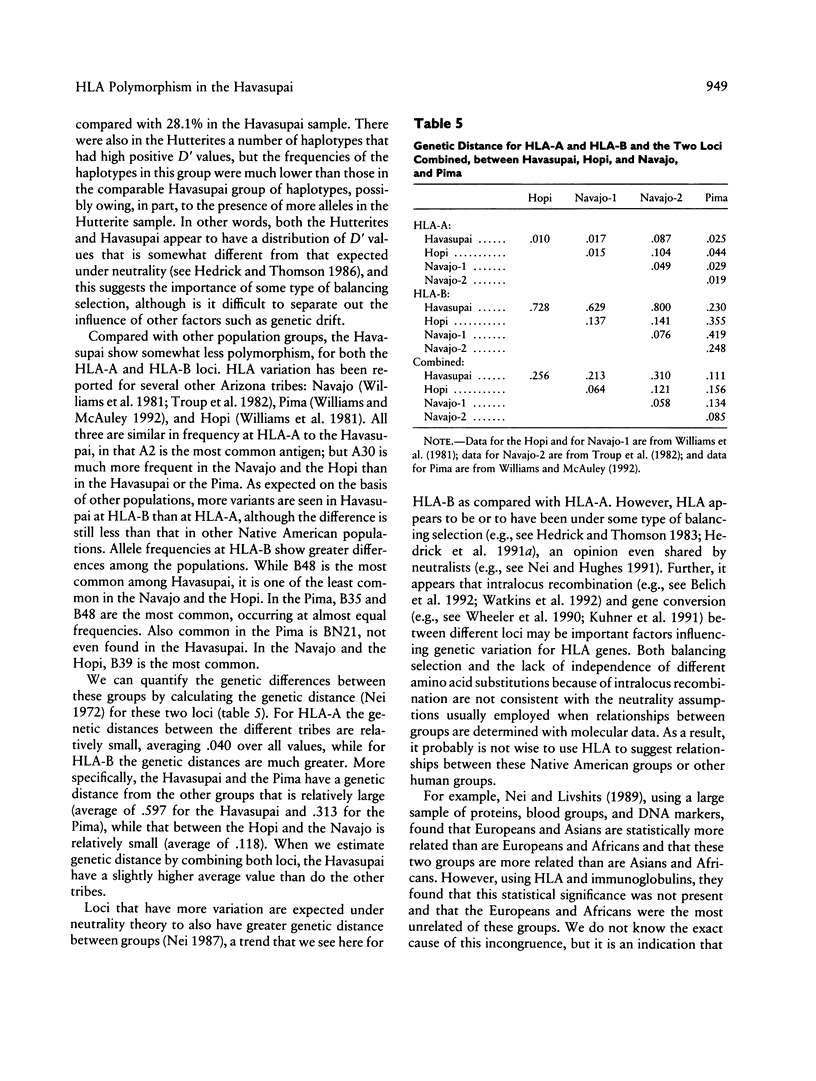
The characterization and analysis of genetic variation at the HLA loci provides important insight for population geneticists trying to understand the evolutionary forces that have shaped human populations. This study describes the HLA-A and HLA-B loci serotyping and statistical analysis on an isolated Native American population, the Havasupai of Arizona. Four alleles at the HLA-A locus were identified, while eight alleles were found at the HLA-B locus. These variants were present as 20 of 32 potential two-locus haplotypes, with five of the six most common haplotypes exhibiting high positive linkage disequilibrium. Significant homozygote deficiency (heterozygosity excess) was detected both at HLA-A and at HLA-B. This deviation from Hardy-Weinberg proportions was not attributable to nonselective causes such as different allele frequencies in males and females or avoidance of consanguineous matings. In addition, the distribution of alleles at both HLA-A and HLA-B was more even than expected from neutrality theory; that is, the observed Hardy-Weinberg homozygosity was only 62.4% of that expected under neutrality. These observations suggest that balancing selection is of major importance in maintaining genetic variation at HLA-A and HLA-B.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belich M. P., Madrigal J. A., Hildebrand W. H., Zemmour J., Williams R. C., Luz R., Petzl-Erler M. L., Parham P. Unusual HLA-B alleles in two tribes of Brazilian Indians. Nature. 1992 May 28;357(6376):326–329. doi: 10.1038/357326a0. [DOI] [PubMed] [Google Scholar]
- Black F. L., Salzano F. M. Evidence for heterosis in the HLA system. Am J Hum Genet. 1981 Nov;33(6):894–899. [PMC free article] [PubMed] [Google Scholar]
- Black F. L. Why did they die? Science. 1992 Dec 11;258(5089):1739–1740. doi: 10.1126/science.1465610. [DOI] [PubMed] [Google Scholar]
- Degos L., Colombani J., Chaventre A., Bengtson B., Jacquard A. Selective pressure on HL-A polymorphism. Nature. 1974 May 3;249(452):62–63. doi: 10.1038/249062a0. [DOI] [PubMed] [Google Scholar]
- Ewens W. J. The sampling theory of selectively neutral alleles. Theor Popul Biol. 1972 Mar;3(1):87–112. doi: 10.1016/0040-5809(72)90035-4. [DOI] [PubMed] [Google Scholar]
- Hedrick P. W. Neutrality or selection at HLA? Am J Hum Genet. 1983 Sep;35(5):1055–1057. [PMC free article] [PubMed] [Google Scholar]
- Hedrick P. W., Thomson G. Evidence for balancing selection at HLA. Genetics. 1983 Jul;104(3):449–456. doi: 10.1093/genetics/104.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick P. W., Whittam T. S., Parham P. Heterozygosity at individual amino acid sites: extremely high levels for HLA-A and -B genes. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5897–5901. doi: 10.1073/pnas.88.13.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Klitz W., Thomson G., Baur M. P. Contrasting evolutionary histories among tightly linked HLA loci. Am J Hum Genet. 1986 Sep;39(3):340–349. [PMC free article] [PubMed] [Google Scholar]
- Klitz W., Thomson G. Disequilibrium pattern analysis. II. Application to Danish HLA A and B locus data. Genetics. 1987 Aug;116(4):633–643. doi: 10.1093/genetics/116.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyu D. D., Ober C. L., Dawson D. V., Ghanayem M., Elias S., Martin A. O. Genetic analysis of HLA in the U.S. Schmiedenleut Hutterites. Am J Hum Genet. 1989 Aug;45(2):261–269. [PMC free article] [PubMed] [Google Scholar]
- Kuhner M. K., Lawlor D. A., Ennis P. D., Parham P. Gene conversion in the evolution of the human and chimpanzee MHC class I loci. Tissue Antigens. 1991 Oct;38(4):152–164. doi: 10.1111/j.1399-0039.1991.tb01889.x. [DOI] [PubMed] [Google Scholar]
- Lewontin R C. The Interaction of Selection and Linkage. I. General Considerations; Heterotic Models. Genetics. 1964 Jan;49(1):49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow T. A., Martin J. F. Inbreeding and developmental stability in a small human population. Ann Hum Biol. 1993 Jul-Aug;20(4):389–394. doi: 10.1080/03014469300002792. [DOI] [PubMed] [Google Scholar]
- Morgan K., Holmes T. M., Pazderka F., Dossetor J. B. Segregation of HLA A,B haplotypes and the distribution of antigen mismatches between mother and offspring in Hutterite families. Am J Hum Genet. 1986 Jun;38(6):971–977. [PMC free article] [PubMed] [Google Scholar]
- Morgan K., Holmes T. M., Schlaut J., Marchuk L., Kovithavongs T., Pazderka F., Dossetor J. B. Genetic variability of HLA in the Dariusleut Hutterites. A comparative genetic analysis of the Hutterities, the Amish, and other selected Caucasian populations. Am J Hum Genet. 1980 Mar;32(2):246–257. [PMC free article] [PubMed] [Google Scholar]
- Nei M., Livshits G. Genetic relationships of Europeans, Asians and Africans and the origin of modern Homo sapiens. Hum Hered. 1989;39(5):276–281. doi: 10.1159/000153872. [DOI] [PubMed] [Google Scholar]
- Ober C., Elias S., Kostyu D. D., Hauck W. W. Decreased fecundability in Hutterite couples sharing HLA-DR. Am J Hum Genet. 1992 Jan;50(1):6–14. [PMC free article] [PubMed] [Google Scholar]
- Potts W. K., Manning C. J., Wakeland E. K. Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature. 1991 Aug 15;352(6336):619–621. doi: 10.1038/352619a0. [DOI] [PubMed] [Google Scholar]
- Purser A. F. Increase in heterozygote frequency with differential fertility. Heredity (Edinb) 1966 May;21(2):322–327. doi: 10.1038/hdy.1966.28. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Bernoco D., Park M. S., Ozturk G., Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol. 1978 Feb;69(2):103–120. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- Thompson E. A., Roberts D. F. Kinship structure and heterozygosity on Tristan da Cunha. Am J Hum Genet. 1980 May;32(3):445–452. [PMC free article] [PubMed] [Google Scholar]
- Thomson G. HLA disease associations: models for insulin dependent diabetes mellitus and the study of complex human genetic disorders. Annu Rev Genet. 1988;22:31–50. doi: 10.1146/annurev.ge.22.120188.000335. [DOI] [PubMed] [Google Scholar]
- Watkins D. I., McAdam S. N., Liu X., Strang C. R., Milford E. L., Levine C. G., Garber T. L., Dogon A. L., Lord C. I., Ghim S. H. New recombinant HLA-B alleles in a tribe of South American Amerindians indicate rapid evolution of MHC class I loci. Nature. 1992 May 28;357(6376):329–333. doi: 10.1038/357329a0. [DOI] [PubMed] [Google Scholar]
- Watterson G. A. The homozygosity test of neutrality. Genetics. 1978 Feb;88(2):405–417. doi: 10.1093/genetics/88.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler C. J., Maloney D., Fogel S., Goodenow R. S. Microconversion between murine H-2 genes integrated into yeast. Nature. 1990 Sep 13;347(6289):192–194. doi: 10.1038/347192a0. [DOI] [PubMed] [Google Scholar]
- Williams R. C., McAuley J. E. HLA class I variation controlled for genetic admixture in the Gila River Indian Community of Arizona: a model for the Paleo-Indians. Hum Immunol. 1992 Jan;33(1):39–46. doi: 10.1016/0198-8859(92)90050-w. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Morse H. G., Bonnell M. D., Rate R. G., Kuberski T. T. The HLA loci of th Hopi and Navajo. Am J Phys Anthropol. 1981 Nov;56(3):291–296. doi: 10.1002/ajpa.1330560309. [DOI] [PubMed] [Google Scholar]
- de Vries R. R., Meera Khan P., Bernini L. F., van Loghem E., van Rood J. J. Genetic control of survival in epidemics. J Immunogenet. 1979 Aug;6(4):271–287. doi: 10.1111/j.1744-313x.1979.tb00684.x. [DOI] [PubMed] [Google Scholar]


