Abstract
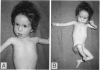
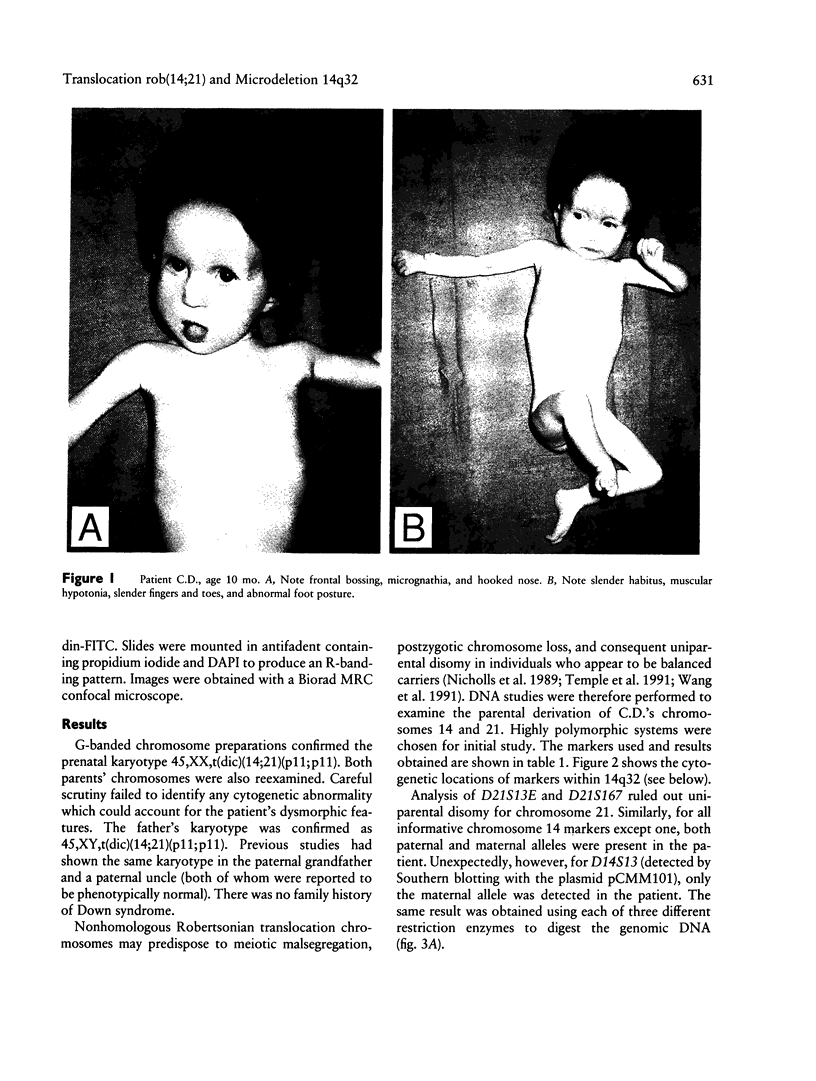
Robertsonian translocations are usually ascertained through abnormal children, making proposed phenotypic effects of apparently balanced translocations difficult to study in an unbiased way. From molecular genetic studies, though, some apparently balanced rearrangements are now known to be associated with phenotypic abnormalities resulting from uniparental disomy. Molecular explanations for other cases in which abnormality is seen in a balanced translocation carrier are being sought. In the present paper, an infant is described who has retarded growth, developmental delay, gross muscular hypotonia, slender habitus, frontal bossing, micrognathia, hooked nose, abundant wispy hair, and blue sclerae. Cytogenetically, she appeared to be a carrier of a balanced, paternally derived 14;21 Robertsonian translocation. Analysis of DNA polymorphisms showed that she had no paternal allele at the D14S13 locus (14q32). Study of additional DNA markers within 14q32 revealed that her previously undescribed phenotype results from an interstitial microdeletion within 14q32. Fluorescent in situ hybridization was used to show that this microdeletion had occurred de novo on the Robertsonian translocation chromosome. These observations may reactivate old suspicions of a causal association between Robertsonian translocations and de novo rearrangements in offspring; a systematic search for similar subcytogenetic rearrangements in other families, in which there are phenotypically abnormal children with apparently balanced translocations, may be fruitful. The clinical and molecular genetic data presented also define a new contiguous gene syndrome due to interstitial 14q32 deletion.
Full text
PDF

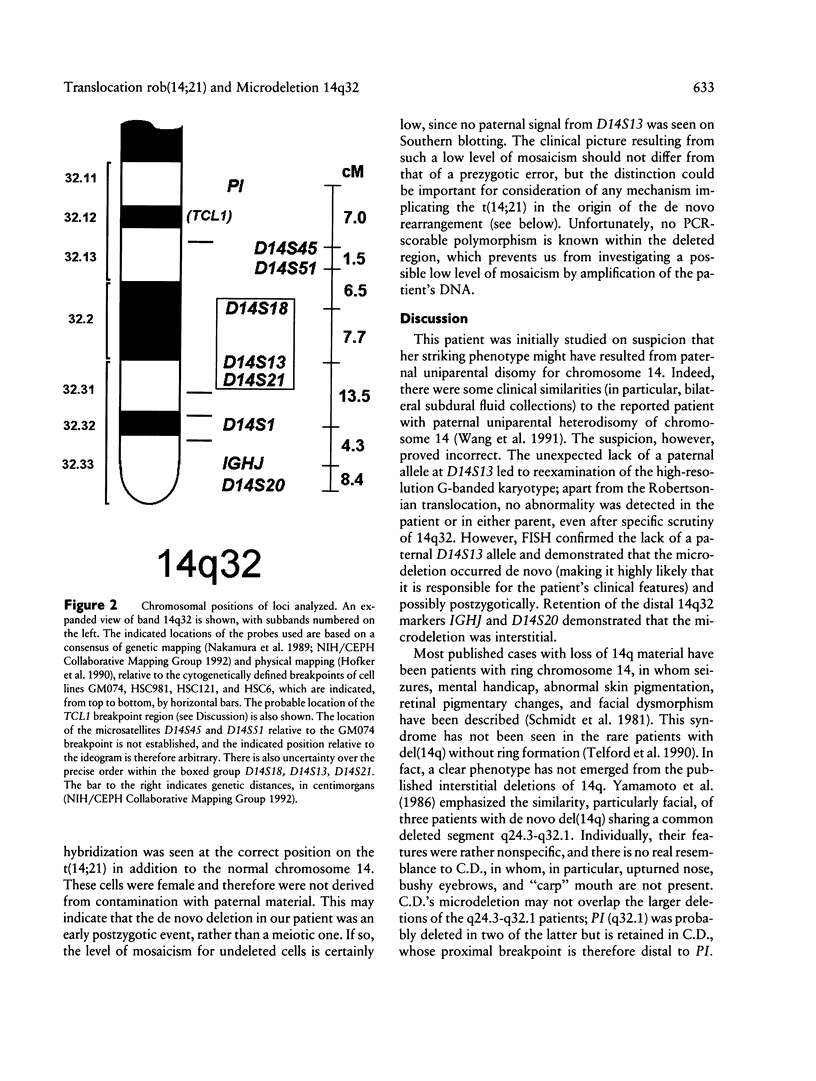
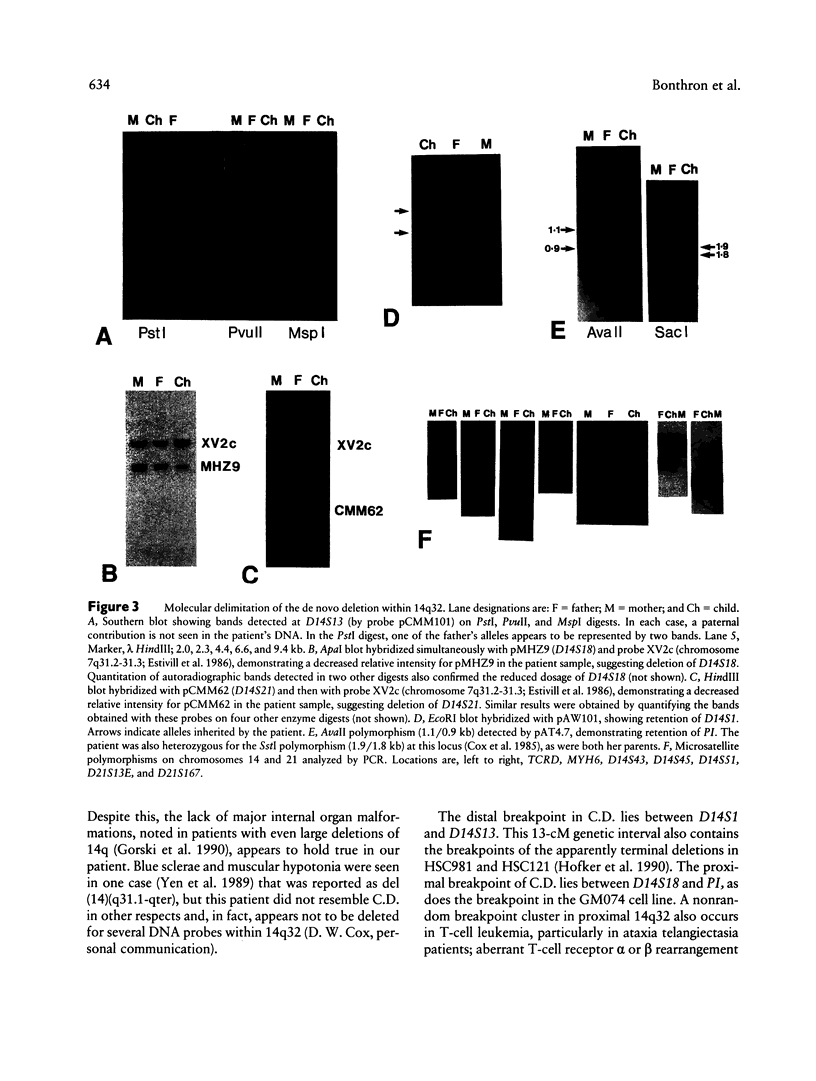
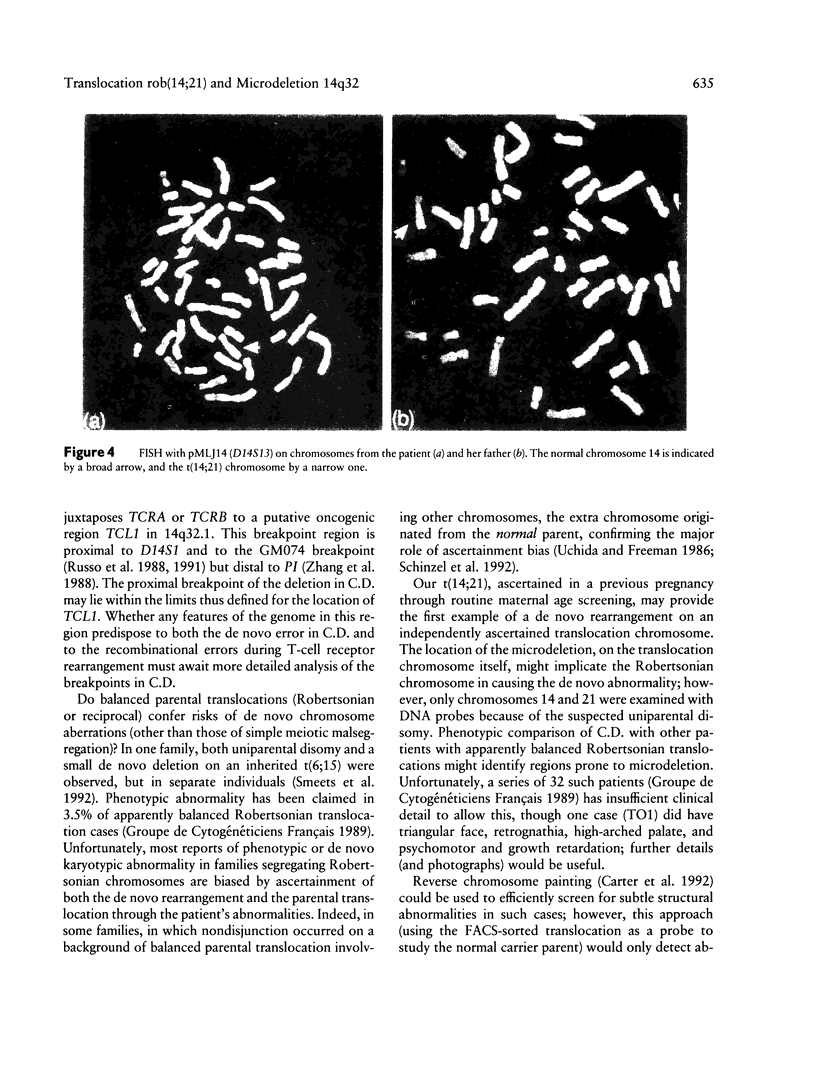


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonthron D., Orkin S. H. The human von Willebrand factor gene. Structure of the 5' region. Eur J Biochem. 1988 Jan 15;171(1-2):51–57. doi: 10.1111/j.1432-1033.1988.tb13757.x. [DOI] [PubMed] [Google Scholar]
- Carter N. P., Ferguson-Smith M. A., Perryman M. T., Telenius H., Pelmear A. H., Leversha M. A., Glancy M. T., Wood S. L., Cook K., Dyson H. M. Reverse chromosome painting: a method for the rapid analysis of aberrant chromosomes in clinical cytogenetics. J Med Genet. 1992 May;29(5):299–307. doi: 10.1136/jmg.29.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Woo S. L., Mansfield T. DNA restriction fragments associated with alpha 1-antitrypsin indicate a single origin for deficiency allele PI Z. Nature. 1985 Jul 4;316(6023):79–81. doi: 10.1038/316079a0. [DOI] [PubMed] [Google Scholar]
- Decorte R., Cuppens H., Marynen P., Cassiman J. J. Rapid detection of hypervariable regions by the polymerase chain reaction technique. DNA Cell Biol. 1990 Jul-Aug;9(6):461–469. doi: 10.1089/dna.1990.9.461. [DOI] [PubMed] [Google Scholar]
- Estivill X., Farrall M., Scambler P. J., Bell G. M., Hawley K. M., Lench N. J., Bates G. P., Kruyer H. C., Frederick P. A., Stanier P. A candidate for the cystic fibrosis locus isolated by selection for methylation-free islands. 1987 Apr 30-May 6Nature. 326(6116):840–845. doi: 10.1038/326840a0. [DOI] [PubMed] [Google Scholar]
- Evans J. A., Canning N., Hunter A. G., Martsolf J. T., Ray M., Thompson D. R., Hamerton J. L. A cytogenetic survey of 14,069 newborn infants. III. an analysis of the significance and cytologic behavior of the Robertsonian and reciprocal translocations. Cytogenet Cell Genet. 1978;20(1-6):96–123. doi: 10.1159/000130843. [DOI] [PubMed] [Google Scholar]
- Fantes J. A., Bickmore W. A., Fletcher J. M., Ballesta F., Hanson I. M., van Heyningen V. Submicroscopic deletions at the WAGR locus, revealed by nonradioactive in situ hybridization. Am J Hum Genet. 1992 Dec;51(6):1286–1294. [PMC free article] [PubMed] [Google Scholar]
- Fougerousse F., Dufour C., Roudaut C., Beckmann J. S. Dinucleotide repeat polymorphism at the human gene for cardiac beta-myosin heavy chain (MYH6). Hum Mol Genet. 1992 Apr;1(1):64–64. doi: 10.1093/hmg/1.1.64-a. [DOI] [PubMed] [Google Scholar]
- Gorski J. L., Uhlmann W. R., Glover T. W. A child with multiple congenital anomalies and karyotype 46,XY,del(14)(q31q32.3): further delineation of chromosome 14 interstitial deletion syndrome. Am J Med Genet. 1990 Dec;37(4):471–474. doi: 10.1002/ajmg.1320370409. [DOI] [PubMed] [Google Scholar]
- Guo Z., Sharma V., Litt M. Dinucleotide repeat polymorphism at the D21S13E locus. Nucleic Acids Res. 1990 Dec 25;18(24):7470–7470. doi: 10.1093/nar/18.24.7470-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Sharma V., Patterson D., Litt M. TG repeat polymorphism at the D21S167 locus. Nucleic Acids Res. 1990 Aug 25;18(16):4967–4967. doi: 10.1093/nar/18.16.4967-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofker M. H., Smith S., Nakamura Y., Teshima I., White R., Cox D. W. Physical mapping of probes within 14q32, a subtelomeric region showing a high recombination frequency. Genomics. 1990 Jan;6(1):33–38. doi: 10.1016/0888-7543(90)90445-z. [DOI] [PubMed] [Google Scholar]
- Jacobs P. A., Melville M., Ratcliffe S., Keay A. J., Syme J. A cytogenetic survey of 11,680 newborn infants. Ann Hum Genet. 1974 May;37(4):359–376. doi: 10.1111/j.1469-1809.1974.tb01843.x. [DOI] [PubMed] [Google Scholar]
- Jordan S. A., McWilliam P., O'Briain D. S., Humphries P. Dinucleotide repeat polymorphism at the T cell receptor delta locus (TCRD). Nucleic Acids Res. 1991 Apr 25;19(8):1959–1959. doi: 10.1093/nar/19.8.1959-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkels V. G., Hustinx T. W., Scheres J. M. Habitual abortion and translocation (22q;22q): unexpected transmission from a mother to her phenotypically normal daughter. Clin Genet. 1980 Dec;18(6):456–461. doi: 10.1111/j.1399-0004.1980.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. H. Minireview: cryptic translocations and telomere integrity. Am J Hum Genet. 1992 Sep;51(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Luty J. A., Litt M. Dinucleotide repeat polymorphism at the D14S45 locus. Nucleic Acids Res. 1991 Aug 11;19(15):4308–4308. doi: 10.1093/nar/19.15.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Nakamura Y., Myers R., Ballard L., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pCMM62) on chromosome 14 [D14S21]. Nucleic Acids Res. 1988 Jun 10;16(11):5220–5220. doi: 10.1093/nar/16.11.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Carlson M., Fujimoto E., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pMCOC12) on chromosome 14 [D14S20]. Nucleic Acids Res. 1988 Jul 11;16(13):6257–6257. doi: 10.1093/nar/16.13.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Culver M., Gill J., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pMLJ14 on chromosome 14 [D14S13]. Nucleic Acids Res. 1988 Jan 11;16(1):381–381. doi: 10.1093/nar/16.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Hoff M., Ballard L., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pMHZ9 on chromosome 14 [D14S18]. Nucleic Acids Res. 1988 Jan 11;16(1):379–379. doi: 10.1093/nar/16.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Lathrop M., O'Connell P., Leppert M., Kamboh M. I., Lalouel J. M., White R. Frequent recombination is observed in the distal end of the long arm of chromosome 14. Genomics. 1989 Jan;4(1):76–81. doi: 10.1016/0888-7543(89)90317-0. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D., Knoll J. H., Butler M. G., Karam S., Lalande M. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989 Nov 16;342(6247):281–285. doi: 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. G., Schwartz S., Hodes M. E. Transmission of a balanced homologous t(22q;22q) translocation from mother to normal daughter. Clin Genet. 1980 Jun;17(6):418–422. doi: 10.1111/j.1399-0004.1980.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Pentao L., Lewis R. A., Ledbetter D. H., Patel P. I., Lupski J. R. Maternal uniparental isodisomy of chromosome 14: association with autosomal recessive rod monochromacy. Am J Hum Genet. 1992 Apr;50(4):690–699. [PMC free article] [PubMed] [Google Scholar]
- Russo G., Isobe M., Gatti R., Finan J., Batuman O., Huebner K., Nowell P. C., Croce C. M. Molecular analysis of a t(14;14) translocation in leukemic T-cells of an ataxia telangiectasia patient. Proc Natl Acad Sci U S A. 1989 Jan;86(2):602–606. doi: 10.1073/pnas.86.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G., Isobe M., Pegoraro L., Finan J., Nowell P. C., Croce C. M. Molecular analysis of a t(7;14)(q35;q32) chromosome translocation in a T cell leukemia of a patient with ataxia telangiectasia. Cell. 1988 Apr 8;53(1):137–144. doi: 10.1016/0092-8674(88)90495-3. [DOI] [PubMed] [Google Scholar]
- Schinzel A. A., Adelsberger P. A., Binkert F., Basaran S., Antonarakis S. E. No evidence for a paternal interchromosomal effect from analysis of the origin of nondisjunction in Down syndrome patients with concomitant familial chromosome rearrangements. Am J Hum Genet. 1992 Feb;50(2):288–293. [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Eviatar L., Nitowsky H. M., Wong M., Miranda S. Ring chromosome 14: a distinct clinical entity. J Med Genet. 1981 Aug;18(4):304–307. doi: 10.1136/jmg.18.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Smith L., Allen L., Magenis R. E., Litt M. Dinucleotide repeat polymorphism at the D14S43 locus. Nucleic Acids Res. 1991 Apr 11;19(7):1722–1722. doi: 10.1093/nar/19.7.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets D. F., Hamel B. C., Nelen M. R., Smeets H. J., Bollen J. H., Smits A. P., Ropers H. H., van Oost B. A. Prader-Willi syndrome and Angelman syndrome in cousins from a family with a translocation between chromosomes 6 and 15. N Engl J Med. 1992 Mar 19;326(12):807–811. doi: 10.1056/NEJM199203193261206. [DOI] [PubMed] [Google Scholar]
- Telford N., Thomson D. A., Griffiths M. J., Ilett S., Watt J. L. Terminal deletion (14)(q32.3): a new case. J Med Genet. 1990 Apr;27(4):261–263. doi: 10.1136/jmg.27.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple I. K., Cockwell A., Hassold T., Pettay D., Jacobs P. Maternal uniparental disomy for chromosome 14. J Med Genet. 1991 Aug;28(8):511–514. doi: 10.1136/jmg.28.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida I. A., Freeman V. C. Trisomy 21 Down syndrome. II. Structural chromosome rearrangements in the parents. Hum Genet. 1986 Feb;72(2):118–122. doi: 10.1007/BF00283928. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Passage M. B., Yen P. H., Shapiro L. J., Mohandas T. K. Uniparental heterodisomy for chromosome 14 in a phenotypically abnormal familial balanced 13/14 Robertsonian translocation carrier. Am J Hum Genet. 1991 Jun;48(6):1069–1074. [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Weber J. L. Continuous linkage map of human chromosome 14 short tandem repeat polymorphisms. Genomics. 1992 Jul;13(3):532–536. doi: 10.1016/0888-7543(92)90121-8. [DOI] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Sawa R., Okamoto N., Matsui A., Yanagisawa M., Ikemoto S. Deletion 14q(q24.3 to q32.1) syndrome: significance of peculiar facial appearance in its diagnosis, and deletion mapping of Pi(alpha 1-antitrypsin). Hum Genet. 1986 Oct;74(2):190–192. doi: 10.1007/BF00282092. [DOI] [PubMed] [Google Scholar]
- Yen F. S., Podruch P. E., Weisskopf B. A terminal deletion (14)(q31.1) in a child with microcephaly, narrow palate, gingival hypertrophy, protuberant ears, and mild mental retardation. J Med Genet. 1989 Feb;26(2):130–133. doi: 10.1136/jmg.26.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Stern M. H., Thomas G., Aurias A. Molecular characterization of ataxia telangiectasia T cell clones. II. The clonal inv(14) in ataxia telangiectasia differs from the inv(14) in T cell lymphoma. Hum Genet. 1988 Apr;78(4):316–319. doi: 10.1007/BF00291726. [DOI] [PubMed] [Google Scholar]