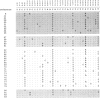
Abstract
Conventional descriptions of the pattern and process of human entry into the New World from Asia are incomplete and controversial. In order to gain an evolutionary insight into this process, we have sequenced the control region of mtDNA in samples of contemporary tribal populations of eastern Siberia, Alaska, and Greenland and have compared them with those of Amerind speakers of the Pacific Northwest and with those of the Altai of central Siberia. Specifically, we have analyzed sequence diversity in 33 mitochondrial lineages identified in 90 individuals belonging to five Circumpolar populations of Beringia, North America, and Greenland: Chukchi from Siberia, Inupiaq Eskimos and Athapaskans from Alaska, Eskimos from West Greenland, and Haida from Canada. Hereafter, we refer to these five populations as "Circumarctic peoples." These data were then compared with the sequence diversity in 47 mitochondrial lineages identified in a sample of 145 individuals from three Amerind-speaking tribes (Bella Coola, Nuu-Chah-Nulth, and Yakima) of the Pacific Northwest, plus 16 mitochondrial lineages identified in a sample of 17 Altai from central Siberia. Sequence diversity within and among Circumarctic populations is considerably less than the sequence diversity observed within and among the three Amerind tribes. The similarity of sequences found among the geographically dispersed Circumarctic groups, plus the small values of mean pairwise sequence differences within Circumarctic populations, suggest a recent and rapid evolutionary radiation of these populations. In addition, Circumarctic populations lack the 9-bp deletion which has been used to trace various migrations out of Asia, while populations of southeastern Siberia possess this deletion. On the basis of these observations, while the evolutionary affinities of Native Americans extend west to the Circumarctic populations of eastern Siberia, they do not include the Altai of central Siberia.
Full text
PDF


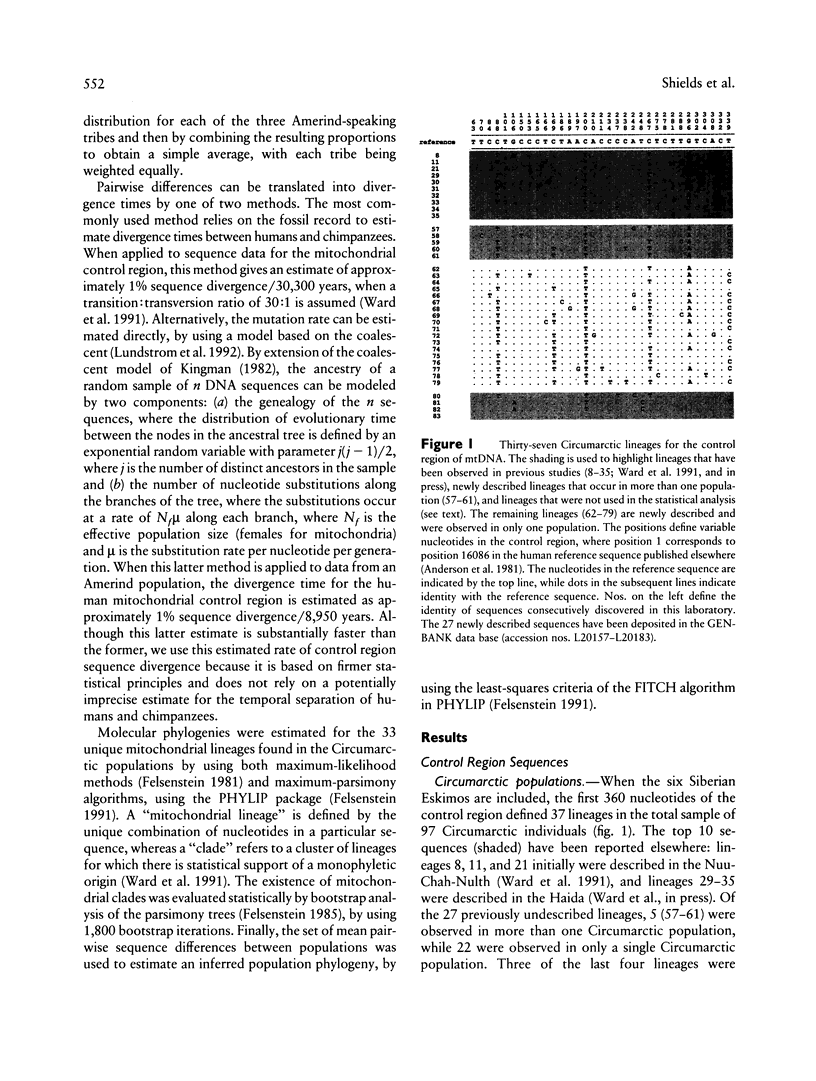
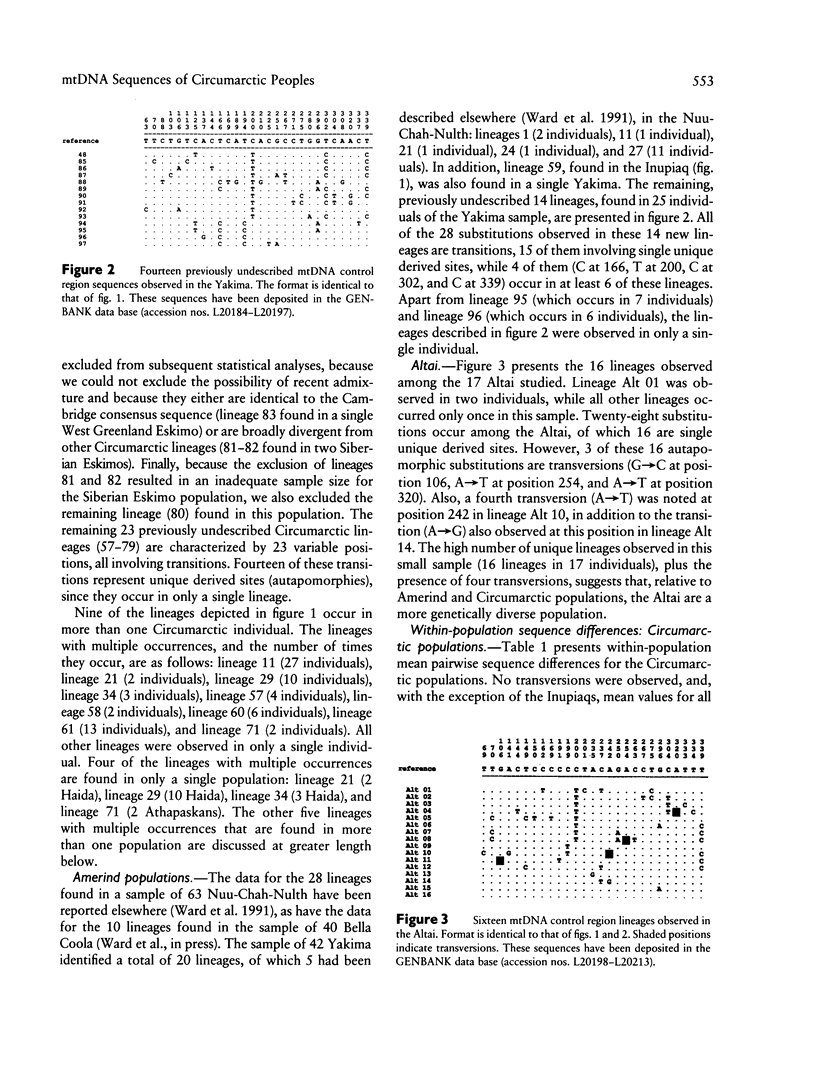





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A. M., Greenberg J. H., Sladky J., Reivich M. Regional variation in cerebral perfusion during acute hypertension. Neurology. 1987 Jan;37(1):94–99. doi: 10.1212/wnl.37.1.94. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Carr S. M., Griffith O. M. Rapid isolation of animal mitochondrial DNA in a small fixed-angle rotor at ultrahigh speed. Biochem Genet. 1987 Jun;25(5-6):385–390. doi: 10.1007/BF00554547. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L. L., Piazza A., Menozzi P., Mountain J. Reconstruction of human evolution: bringing together genetic, archaeological, and linguistic data. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6002–6006. doi: 10.1073/pnas.85.16.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzo A., Wilson A. C. Branching pattern in the evolutionary tree for human mitochondrial DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1597–1601. doi: 10.1073/pnas.88.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Ferrell R. E., Chakraborty R., Gershowitz H., Laughlin W. S., Schull W. J. The St. Lawrence Island Eskimos: genetic variation and genetic distance. Am J Phys Anthropol. 1981 Jul;55(3):351–358. doi: 10.1002/ajpa.1330550309. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harihara S., Hirai M., Suutou Y., Shimizu K., Omoto K. Frequency of a 9-bp deletion in the mitochondrial DNA among Asian populations. Hum Biol. 1992 Apr;64(2):161–166. [PubMed] [Google Scholar]
- Harper A. B. Origins and divergence of Aleuts, Eskimos, and American Indians. Ann Hum Biol. 1980 Nov-Dec;7(6):547–554. doi: 10.1080/03014468000004661. [DOI] [PubMed] [Google Scholar]
- Hertzberg M., Mickleson K. N., Serjeantson S. W., Prior J. F., Trent R. J. An Asian-specific 9-bp deletion of mitochondrial DNA is frequently found in Polynesians. Am J Hum Genet. 1989 Apr;44(4):504–510. [PMC free article] [PubMed] [Google Scholar]
- Hey J. The structure of genealogies and the distribution of fixed differences between DNA sequence samples from natural populations. Genetics. 1991 Aug;128(4):831–840. doi: 10.1093/genetics/128.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffecker J. F., Powers W. R., Goebel T. The colonization of beringia and the peopling of the new world. Science. 1993 Jan 1;259(5091):46–53. doi: 10.1126/science.259.5091.46. [DOI] [PubMed] [Google Scholar]
- Horai S., Kondo R., Nakagawa-Hattori Y., Hayashi S., Sonoda S., Tajima K. Peopling of the Americas, founded by four major lineages of mitochondrial DNA. Mol Biol Evol. 1993 Jan;10(1):23–47. doi: 10.1093/oxfordjournals.molbev.a039987. [DOI] [PubMed] [Google Scholar]
- Kimura M. Estimation of evolutionary distances between homologous nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Jan;78(1):454–458. doi: 10.1073/pnas.78.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher T. D., Thomas W. K., Meyer A., Edwards S. V., Päbo S., Villablanca F. X., Wilson A. C. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom R., Tavaré S., Ward R. H. Estimating substitution rates from molecular data using the coalescent. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5961–5965. doi: 10.1073/pnas.89.13.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sambuugiin N., Petrishchev V. N., Rychkov Iu G. Polimorfizm DNK v naselenii Mongolii. Analiz PDRF mitokhondrial'noi DNK. Genetika. 1991 Dec;27(12):2143–2151. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanfield M. S., Alexeyeva T. E., Crawford M. H. Studies on the immunoglobulin allotypes of asiatic populations. VIII. Immunoglobulin allotypes among the Tuvinians of the USSR. Hum Hered. 1980;30(6):343–349. doi: 10.1159/000153155. [DOI] [PubMed] [Google Scholar]
- Schell L. M., Agarwal S. S., Blumberg B. S., Levy H., Bennett P. H., Laughlin W. S., Martin J. P. Distribution of albumin variants Naskapi amd Mexico among Aleuts, Frobisher Bay Eskimos, and Micmac, Naskapi, Mohawk, Omaha, and Apache Indians. Am J Phys Anthropol. 1978 Jul;49(1):111–117. doi: 10.1002/ajpa.1330490117. [DOI] [PubMed] [Google Scholar]
- Schurr T. G., Ballinger S. W., Gan Y. Y., Hodge J. A., Merriwether D. A., Lawrence D. N., Knowler W. C., Weiss K. M., Wallace D. C. Amerindian mitochondrial DNAs have rare Asian mutations at high frequencies, suggesting they derived from four primary maternal lineages. Am J Hum Genet. 1990 Mar;46(3):613–623. [PMC free article] [PubMed] [Google Scholar]
- Shields G. F., Hecker K., Voevoda M. I., Reed J. K. Absence of the Asian-specific region V mitochondrial marker in Native Beringians. Am J Hum Genet. 1992 Apr;50(4):758–765. [PMC free article] [PubMed] [Google Scholar]
- Shields G. F., Wilson A. C. Calibration of mitochondrial DNA evolution in geese. J Mol Evol. 1987;24(3):212–217. doi: 10.1007/BF02111234. [DOI] [PubMed] [Google Scholar]
- Stoneking M., Bhatia K., Wilson A. C. Rate of sequence divergence estimated from restriction maps of mitochondrial DNAs from Papua New Guinea. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):433–439. doi: 10.1101/sqb.1986.051.01.052. [DOI] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Yang C. C., Szathmary E. J., Williams R. C., Schanfield M. S., Troup G. A., Knowler W. C., Lawrence D. N., Weiss K. M. Native American mitochondrial DNA analysis indicates that the Amerind and the Nadene populations were founded by two independent migrations. Genetics. 1992 Jan;130(1):153–162. doi: 10.1093/genetics/130.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Pennington R., Harpending H., Kocher T. D., Wilson A. C. Mitochondrial DNA sequences in single hairs from a southern African population. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9350–9354. doi: 10.1073/pnas.86.23.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Garrison K., Knowler W. C. Dramatic founder effects in Amerindian mitochondrial DNAs. Am J Phys Anthropol. 1985 Oct;68(2):149–155. doi: 10.1002/ajpa.1330680202. [DOI] [PubMed] [Google Scholar]
- Ward R. H., Frazier B. L., Dew-Jager K., Päbo S. Extensive mitochondrial diversity within a single Amerindian tribe. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8720–8724. doi: 10.1073/pnas.88.19.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]