Abstract
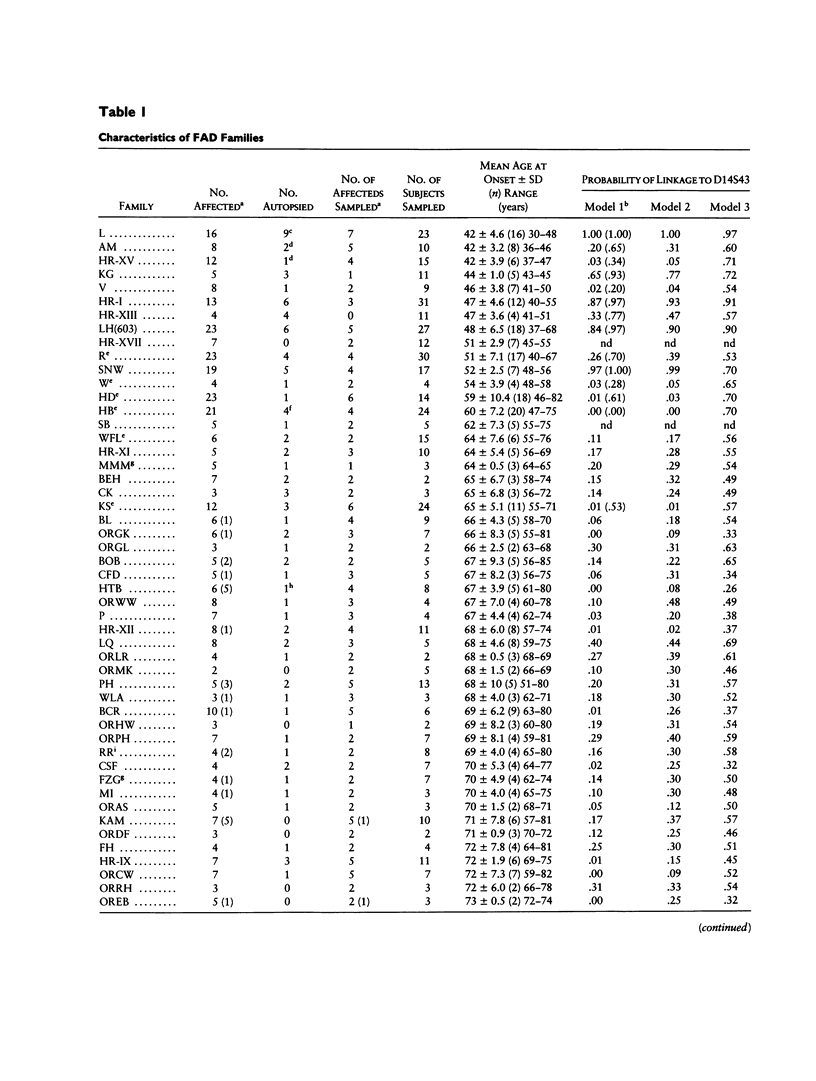
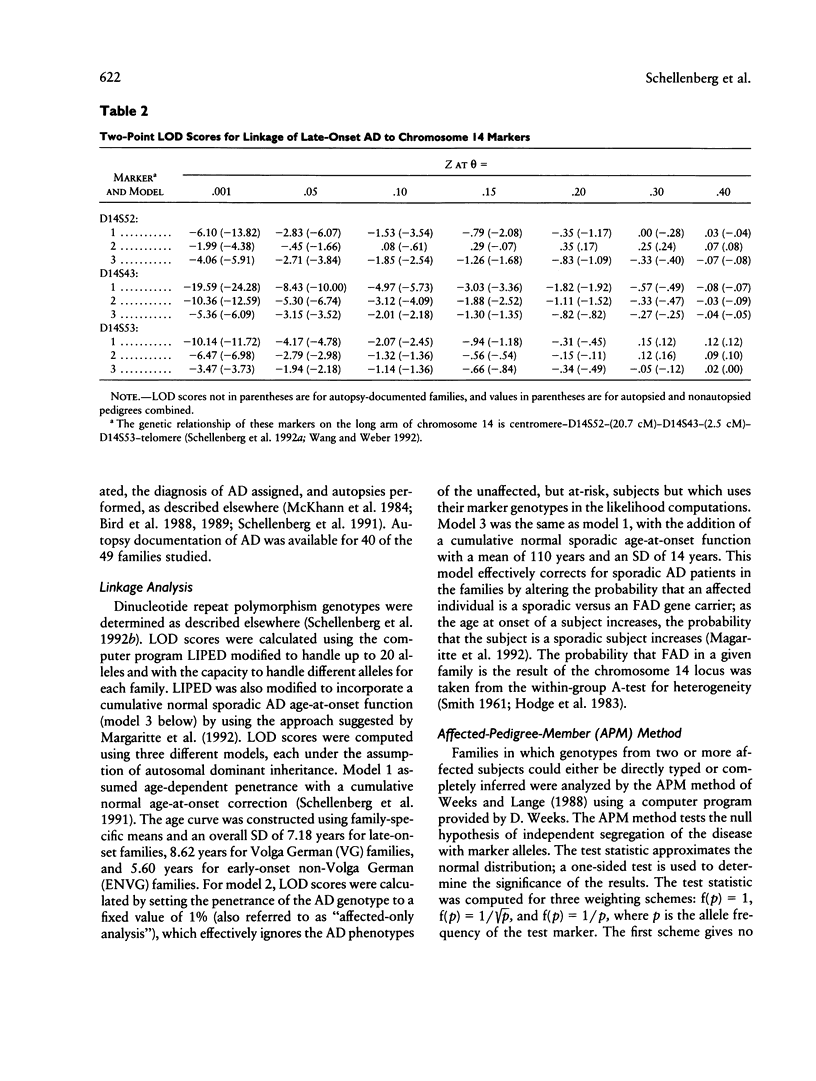
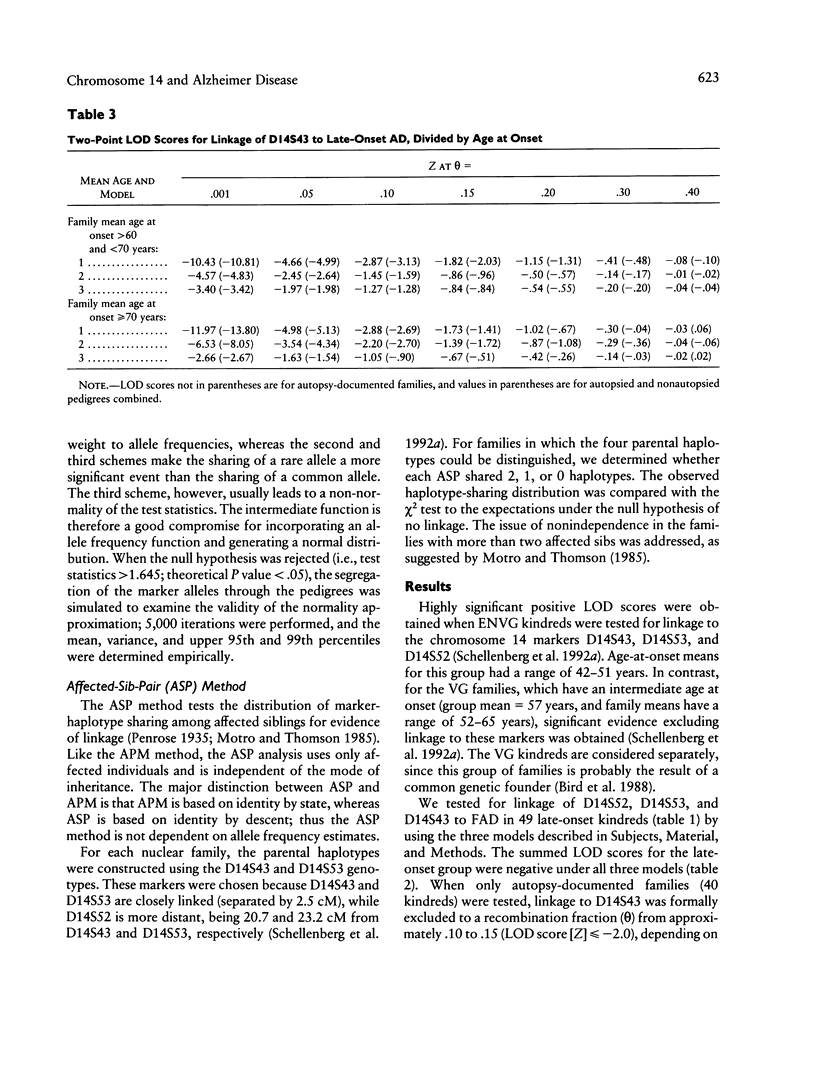
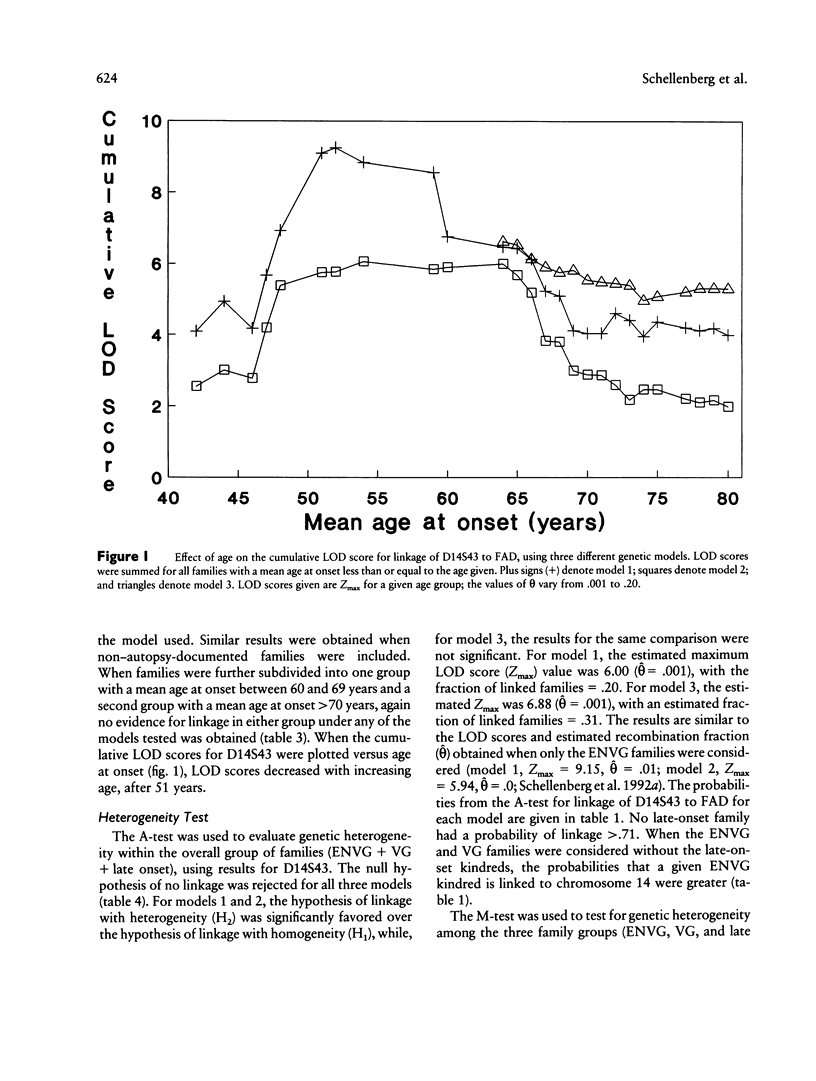
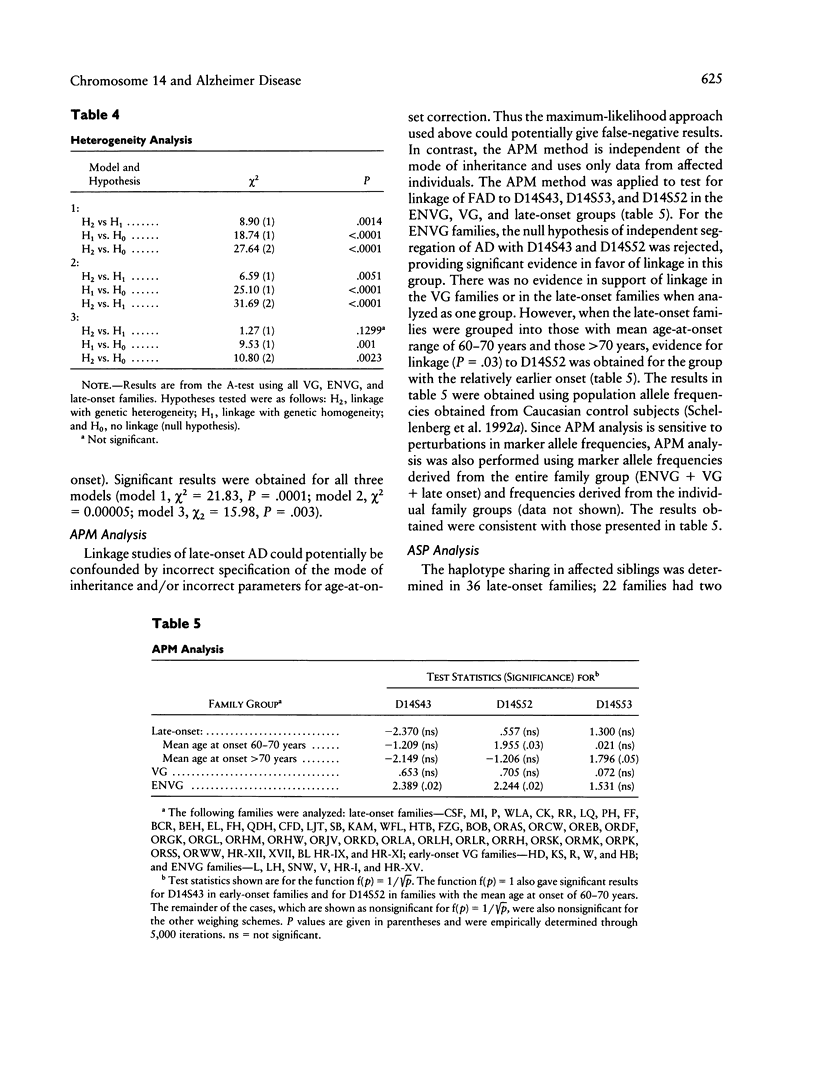
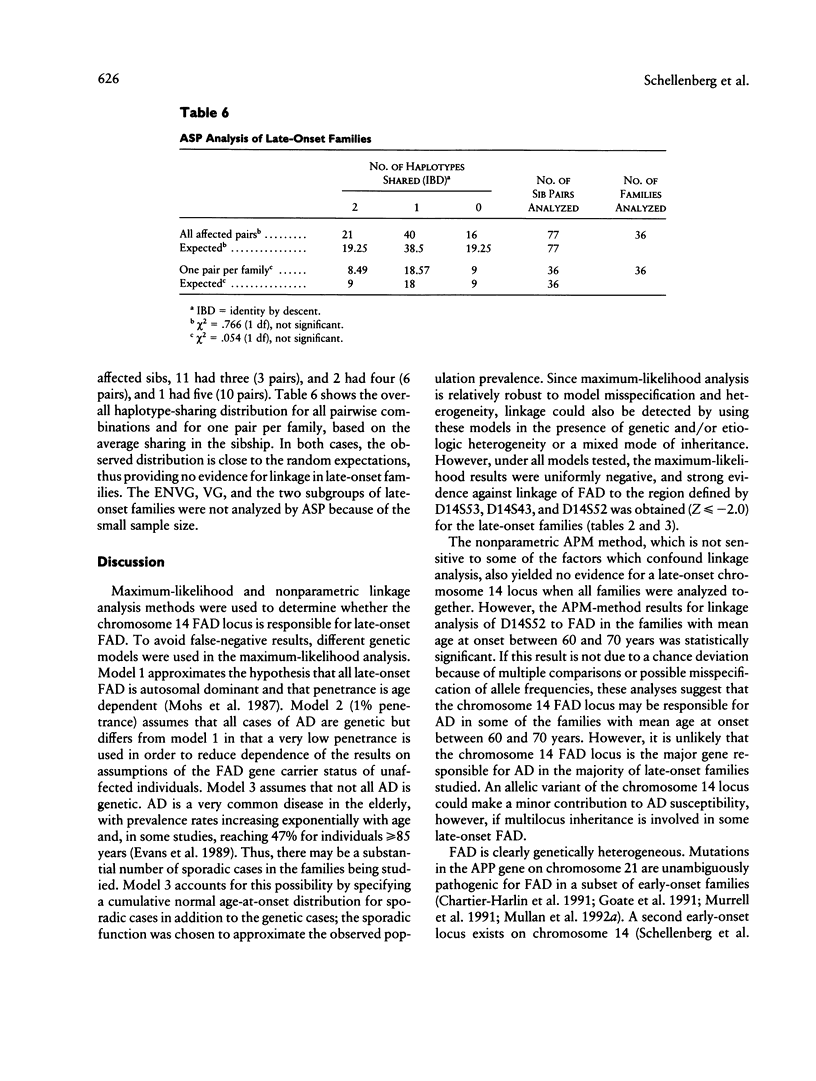
Familial Alzheimer disease (FAD) is genetically heterogeneous. Two loci responsible for early-onset FAD have been identified: the amyloid precursor protein gene on chromosome 21 and the as-yet-unidentified locus on chromosome 14. The genetics of late-onset FAD is unresolved. Maximum-likelihood, affected-pedigree-member (APM), and sib-pair analyses were used, in 49 families with a mean age at onset ≥60 years, to determine whether the chromosome 14 locus is responsible for late-onset FAD. The markers used were D14S53, D14S43, and D14S52. The LOD score method was used to test for linkage of late-onset FAD to the chromosome 14 markers, under three different models: age-dependent penetrance, an affected-only analysis, and age-dependent penetrance with allowance for possible age-dependent sporadic cases. No evidence for linkage was obtained under any of these conditions for the late-onset kindreds, and strong evidence against linkage (LOD score ≤ –2.0) to this region was obtained. Heterogeneity tests of the LOD score results for the combined group of families (early onset, Volga Germans, and late onset) favored the hypothesis of linkage to chromosome 14 with genetic heterogeneity. The positive results are primarily from early-onset families. APM analysis gave significant evidence for linkage of D14S43 and D14S52 to FAD in early-onset kindreds (P < .02). No evidence for linkage was found for the entire late-onset family group. Significant evidence for linkage to D14S52, however, was found for a subgroup of families of intermediate age at onset (mean age at onset ≥60 years and <70 years). These results indicate that the chromosome 14 locus is not responsible for Alzheimer disease in most late-onset FAD kindreds but could play a role in a subset of these kindreds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird T. D., Lampe T. H., Nemens E. J., Miner G. W., Sumi S. M., Schellenberg G. D. Familial Alzheimer's disease in American descendants of the Volga Germans: probable genetic founder effect. Ann Neurol. 1988 Jan;23(1):25–31. doi: 10.1002/ana.410230106. [DOI] [PubMed] [Google Scholar]
- Bird T. D., Sumi S. M., Nemens E. J., Nochlin D., Schellenberg G., Lampe T. H., Sadovnick A., Chui H., Miner G. W., Tinklenberg J. Phenotypic heterogeneity in familial Alzheimer's disease: a study of 24 kindreds. Ann Neurol. 1989 Jan;25(1):12–25. doi: 10.1002/ana.410250104. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991 Oct 31;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Funkenstein H. H., Albert M. S., Scherr P. A., Cook N. R., Chown M. J., Hebert L. E., Hennekens C. H., Taylor J. O. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989 Nov 10;262(18):2551–2556. [PubMed] [Google Scholar]
- Farrer L. A., Myers R. H., Connor L., Cupples L. A., Growdon J. H. Segregation analysis reveals evidence of a major gene for Alzheimer disease. Am J Hum Genet. 1991 Jun;48(6):1026–1033. [PMC free article] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hodge S. E., Anderson C. E., Neiswanger K., Sparkes R. S., Rimoin D. L. The search for heterogeneity in insulin-dependent diabetes mellitus (IDDM): linkage studies, two-locus models, and genetic heterogeneity. Am J Hum Genet. 1983 Nov;35(6):1139–1155. [PMC free article] [PubMed] [Google Scholar]
- Kamino K., Orr H. T., Payami H., Wijsman E. M., Alonso M. E., Pulst S. M., Anderson L., O'dahl S., Nemens E., White J. A. Linkage and mutational analysis of familial Alzheimer disease kindreds for the APP gene region. Am J Hum Genet. 1992 Nov;51(5):998–1014. [PMC free article] [PubMed] [Google Scholar]
- Margaritte P., Bonaiti-Pellie C., King M. C., Clerget-Darpoux F. Linkage of familial breast cancer to chromosome 17q21 may not be restricted to early-onset disease. Am J Hum Genet. 1992 Jun;50(6):1231–1234. [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mohs R. C., Breitner J. C., Silverman J. M., Davis K. L. Alzheimer's disease. Morbid risk among first-degree relatives approximates 50% by 90 years of age. Arch Gen Psychiatry. 1987 May;44(5):405–408. doi: 10.1001/archpsyc.1987.01800170019003. [DOI] [PubMed] [Google Scholar]
- Motro U., Thomson G. The affected sib method. I. Statistical features of the affected sib-pair method. Genetics. 1985 Jul;110(3):525–538. doi: 10.1093/genetics/110.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Mullan M., Houlden H., Windelspecht M., Fidani L., Lombardi C., Diaz P., Rossor M., Crook R., Hardy J., Duff K. A locus for familial early-onset Alzheimer's disease on the long arm of chromosome 14, proximal to the alpha 1-antichymotrypsin gene. Nat Genet. 1992 Dec;2(4):340–342. doi: 10.1038/ng1292-340. [DOI] [PubMed] [Google Scholar]
- Murrell J., Farlow M., Ghetti B., Benson M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance M. A., Bebout J. L., Gaskell P. C., Jr, Yamaoka L. H., Hung W. Y., Alberts M. J., Walker A. P., Bartlett R. J., Haynes C. A., Welsh K. A. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991 Jun;48(6):1034–1050. [PMC free article] [PubMed] [Google Scholar]
- Pericak-Vance M. A., Yamaoka L. H., Haynes C. S., Speer M. C., Haines J. L., Gaskell P. C., Hung W. Y., Clark C. M., Heyman A. L., Trofatter J. A. Genetic linkage studies in Alzheimer's disease families. Exp Neurol. 1988 Dec;102(3):271–279. doi: 10.1016/0014-4886(88)90220-8. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Pettigrew K. D., Schapiro M. B. Discordance and concordance of dementia of the Alzheimer type (DAT) in monozygotic twins indicate heritable and sporadic forms of Alzheimer's disease. Neurology. 1991 Oct;41(10):1549–1553. doi: 10.1212/wnl.41.10.1549. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Moore D. K., Boehnke M., Bryant E. M., Lampe T. H., Nochlin D., Sumi S. M., Deeb S. S. Absence of linkage of chromosome 21q21 markers to familial Alzheimer's disease. Science. 1988 Sep 16;241(4872):1507–1510. doi: 10.1126/science.3420406. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Orr H. T., Anderson L., Nemens E., White J. A., Bonnycastle L., Weber J. L., Alonso M. E. Genetic linkage evidence for a familial Alzheimer's disease locus on chromosome 14. Science. 1992 Oct 23;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Boehnke M., Wijsman E. M., Moore D. K., Martin G. M., Bird T. D. Genetic association and linkage analysis of the apolipoprotein CII locus and familial Alzheimer's disease. Ann Neurol. 1992 Feb;31(2):223–227. doi: 10.1002/ana.410310214. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Deeb S. S., Boehnke M., Bryant E. M., Martin G. M., Lampe T. H., Bird T. D. Association of an apolipoprotein CII allele with familial dementia of the Alzheimer type. J Neurogenet. 1987 Apr;4(2-3):97–108. [PubMed] [Google Scholar]
- Schellenberg G. D., Pericak-Vance M. A., Wijsman E. M., Moore D. K., Gaskell P. C., Jr, Yamaoka L. A., Bebout J. L., Anderson L., Welsh K. A., Clark C. M. Linkage analysis of familial Alzheimer disease, using chromosome 21 markers. Am J Hum Genet. 1991 Mar;48(3):563–583. [PMC free article] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Haines J. L., Farrer L. A., Polinsky R., Van Broeckhoven C., Goate A., McLachlan D. R., Orr H., Bruni A. C., Sorbi S. Genetic linkage studies suggest that Alzheimer's disease is not a single homogeneous disorder. Nature. 1990 Sep 13;347(6289):194–197. doi: 10.1038/347194a0. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Tanzi R. E., Polinsky R. J., Haines J. L., Nee L., Watkins P. C., Myers R. H., Feldman R. G., Pollen D., Drachman D. The genetic defect causing familial Alzheimer's disease maps on chromosome 21. Science. 1987 Feb 20;235(4791):885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P., Haines J., Rogaev E., Mortilla M., Vaula G., Pericak-Vance M., Foncin J. F., Montesi M., Bruni A., Sorbi S. Genetic evidence for a novel familial Alzheimer's disease locus on chromosome 14. Nat Genet. 1992 Dec;2(4):330–334. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., George-Hyslop P. S., Gusella J. F. Molecular genetics of Alzheimer disease amyloid. J Biol Chem. 1991 Nov 5;266(31):20579–20582. [PubMed] [Google Scholar]
- Van Broeckhoven C., Backhovens H., Cruts M., De Winter G., Bruyland M., Cras P., Martin J. J. Mapping of a gene predisposing to early-onset Alzheimer's disease to chromosome 14q24.3. Nat Genet. 1992 Dec;2(4):335–339. doi: 10.1038/ng1292-335. [DOI] [PubMed] [Google Scholar]
- Wang Z., Weber J. L. Continuous linkage map of human chromosome 14 short tandem repeat polymorphisms. Genomics. 1992 Jul;13(3):532–536. doi: 10.1016/0888-7543(92)90121-8. [DOI] [PubMed] [Google Scholar]
- Weeks D. E., Lange K. The affected-pedigree-member method of linkage analysis. Am J Hum Genet. 1988 Feb;42(2):315–326. [PMC free article] [PubMed] [Google Scholar]
- van Duijn C. M., Clayton D., Chandra V., Fratiglioni L., Graves A. B., Heyman A., Jorm A. F., Kokmen E., Kondo K., Mortimer J. A. Familial aggregation of Alzheimer's disease and related disorders: a collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20 (Suppl 2):S13–S20. doi: 10.1093/ije/20.supplement_2.s13. [DOI] [PubMed] [Google Scholar]


