Abstract
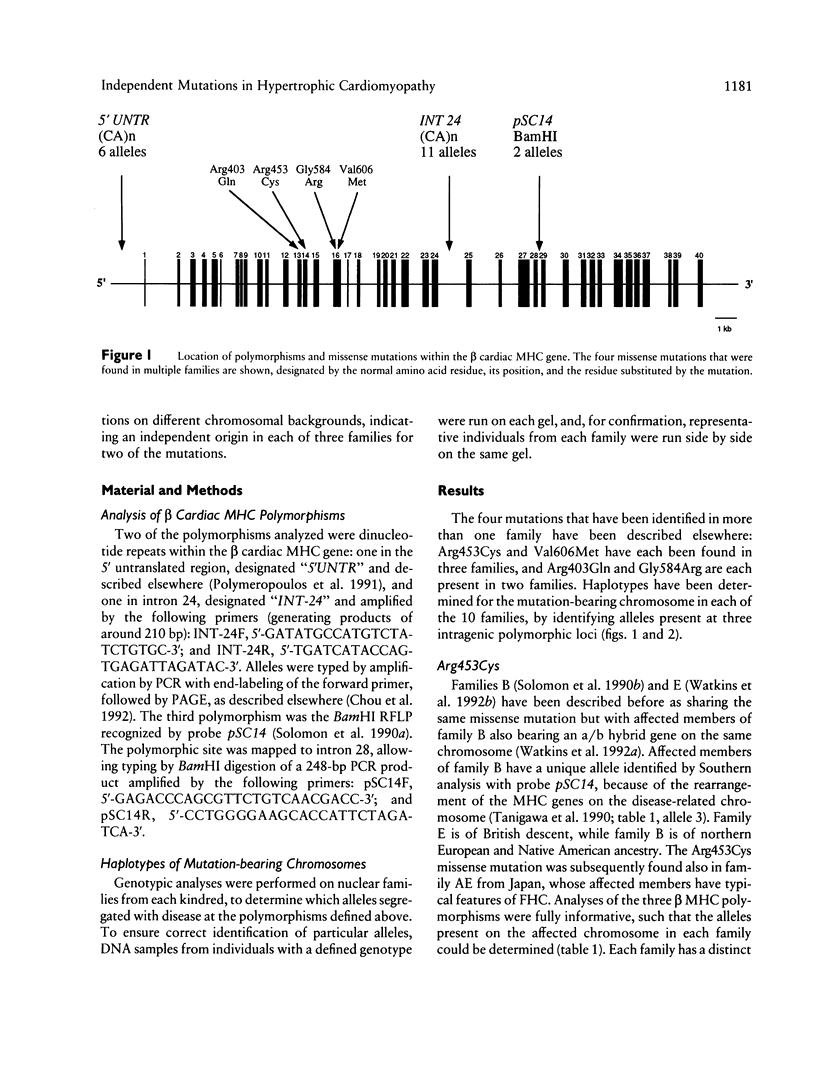
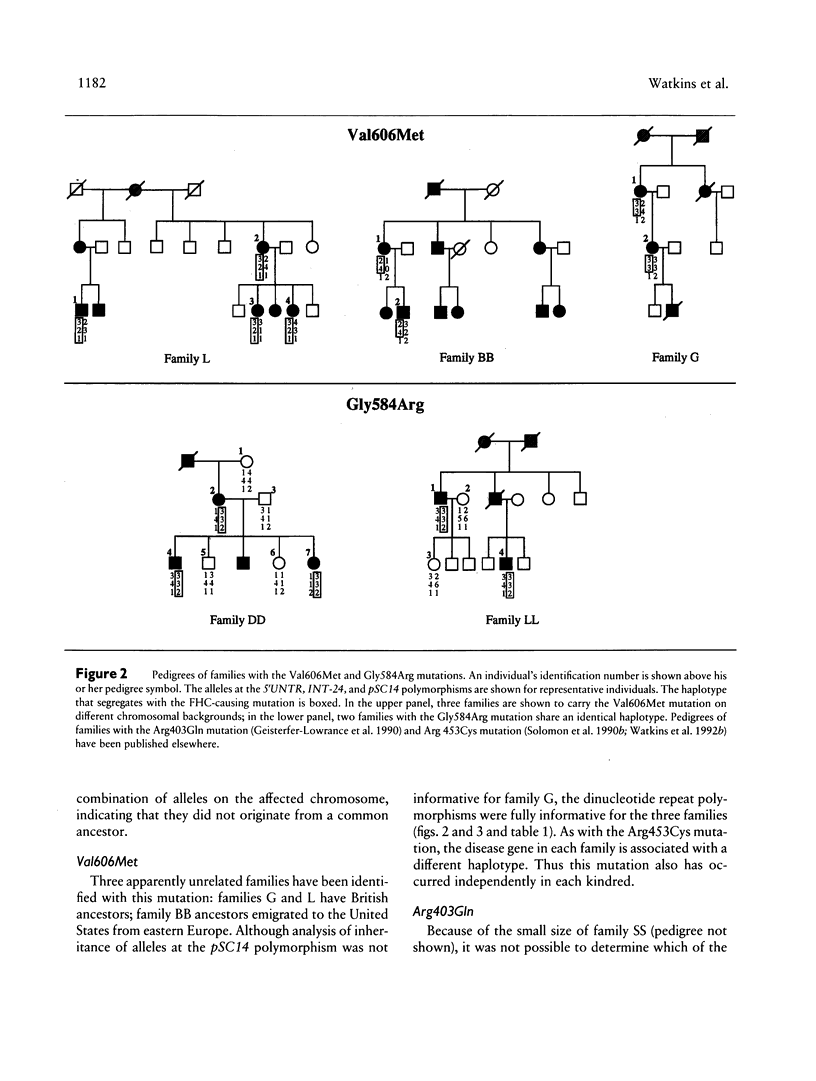
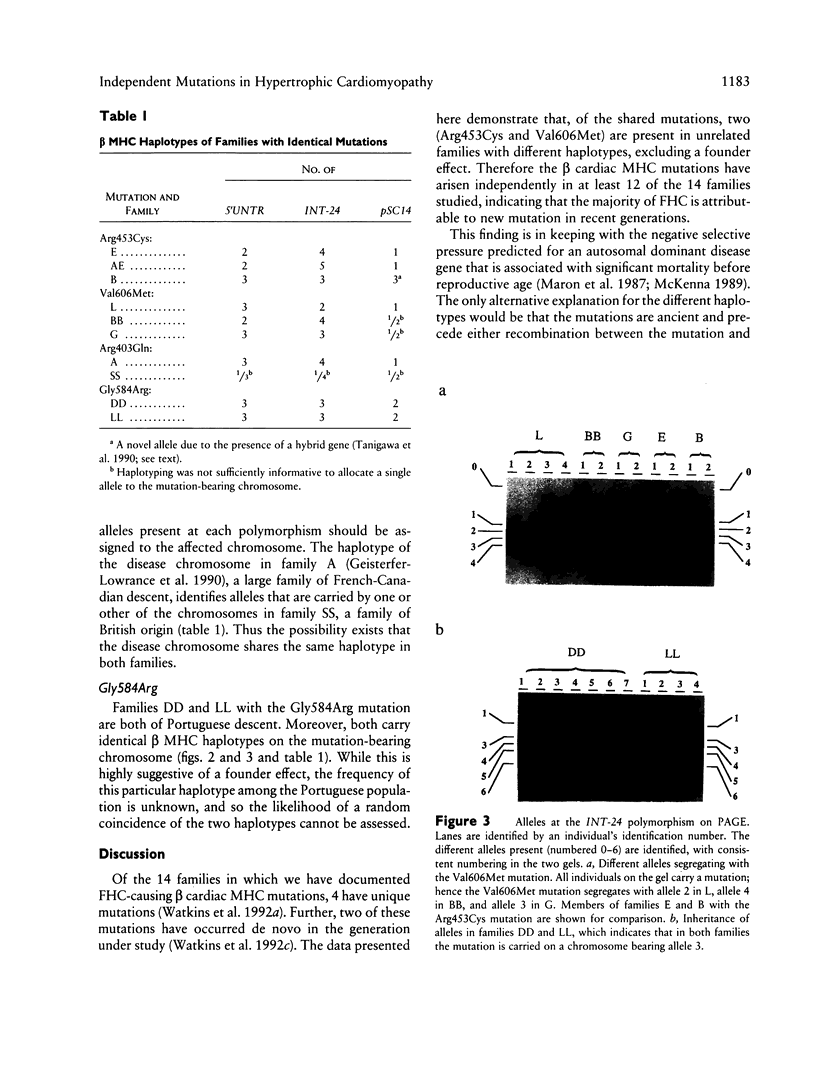
The origins of the beta cardiac myosin heavy-chain (MHC) gene missense mutations that cause familial hypertrophic cardiomyopathy (FHC) in 14 families have been evaluated. Of eight different mutations, four were present in single families, while four occurred in two or more families. To investigate the origins of the four shared mutations, we defined the beta cardiac MHC haplotypes of each of the mutation-bearing chromosomes by determining the alleles present at three intragenic polymorphic loci. Two of the mutations (Arg453Cys and Val606Met) have arisen independently in each of three families, being found on different chromosomal backgrounds. A third mutation (Gly584Arg) is associated with identical haplotypes in two families with Portuguese ancestors, suggesting a founder effect. Haplotype analysis was uninformative for the fourth mutation (Arg403Gln). Thus, FHC-causing mutations have arisen independently in at least 12 of the 14 families studied, suggesting that the majority have arisen relatively recently as new mutations. This finding predicts the prevalence of disease-causing beta cardiac MHC mutations to be comparable in all population groups.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bejsovec A., Anderson P. Functions of the myosin ATP and actin binding sites are required for C. elegans thick filament assembly. Cell. 1990 Jan 12;60(1):133–140. doi: 10.1016/0092-8674(90)90723-r. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Brown E. M., Levi T., Crowe G., Atkinson A. B., Arnqvist H. J., Toss G., Fuleihan G. E., Seidman J. G., Seidman C. E. The gene responsible for familial hypocalciuric hypercalcemia maps to chromosome 3q in four unrelated families. Nat Genet. 1992 Jul;1(4):295–300. doi: 10.1038/ng0792-295. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein N. D., Cohn G. M., Cyran F., Fananapazir L. Differences in clinical expression of hypertrophic cardiomyopathy associated with two distinct mutations in the beta-myosin heavy chain gene. A 908Leu----Val mutation and a 403Arg----Gln mutation. Circulation. 1992 Aug;86(2):345–352. doi: 10.1161/01.cir.86.2.345. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance A. A., Kass S., Tanigawa G., Vosberg H. P., McKenna W., Seidman C. E., Seidman J. G. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990 Sep 7;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- Green P. M., Montandon A. J., Bentley D. R., Ljung R., Nilsson I. M., Giannelli F. The incidence and distribution of CpG----TpG transitions in the coagulation factor IX gene. A fresh look at CpG mutational hotspots. Nucleic Acids Res. 1990 Jun 11;18(11):3227–3231. doi: 10.1093/nar/18.11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik J. F., Brink P. A., Towbin J., Hill R., Brink L., Tapscott T., Trakhtenbroit A., Roberts R. Localization of gene for familial hypertrophic cardiomyopathy to chromosome 14q1 in a diverse US population. Circulation. 1991 May;83(5):1592–1597. doi: 10.1161/01.cir.83.5.1592. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Bonow R. O., Cannon R. O., 3rd, Leon M. B., Epstein S. E. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (1). N Engl J Med. 1987 Mar 26;316(13):780–789. doi: 10.1056/NEJM198703263161305. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Xiao H., Rath D. S., Merril C. R. Dinucleotide repeat polymorphism at the human cardiac beta-myosin gene. Nucleic Acids Res. 1991 Jul 25;19(14):4019–4019. [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig A., Watkins H., Hwang D. S., Miri M., McKenna W., Traill T. A., Seidman J. G., Seidman C. E. Preclinical diagnosis of familial hypertrophic cardiomyopathy by genetic analysis of blood lymphocytes. N Engl J Med. 1991 Dec 19;325(25):1753–1760. doi: 10.1056/NEJM199112193252501. [DOI] [PubMed] [Google Scholar]
- Schwartz K., Beckmann J., Dufour C., Faure L., Fougerousse F., Carrier L., Hengstenberg C., Cohen D., Vosberg H. P., Sacrez A. Exclusion of cardiac myosin heavy chain and actin gene involvement in hypertrophic cardiomyopathy of several French families. Circ Res. 1992 Jul;71(1):3–8. doi: 10.1161/01.res.71.1.3. [DOI] [PubMed] [Google Scholar]
- Solomon S. D., Geisterfer-Lowrance A. A., Vosberg H. P., Hiller G., Jarcho J. A., Morton C. C., McBride W. O., Mitchell A. L., Bale A. E., McKenna W. J. A locus for familial hypertrophic cardiomyopathy is closely linked to the cardiac myosin heavy chain genes, CRI-L436, and CRI-L329 on chromosome 14 at q11-q12. Am J Hum Genet. 1990 Sep;47(3):389–394. [PMC free article] [PubMed] [Google Scholar]
- Solomon S. D., Jarcho J. A., McKenna W., Geisterfer-Lowrance A., Germain R., Salerni R., Seidman J. G., Seidman C. E. Familial hypertrophic cardiomyopathy is a genetically heterogeneous disease. J Clin Invest. 1990 Sep;86(3):993–999. doi: 10.1172/JCI114802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa G., Jarcho J. A., Kass S., Solomon S. D., Vosberg H. P., Seidman J. G., Seidman C. E. A molecular basis for familial hypertrophic cardiomyopathy: an alpha/beta cardiac myosin heavy chain hybrid gene. Cell. 1990 Sep 7;62(5):991–998. doi: 10.1016/0092-8674(90)90273-h. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhou J., Todorovic S. M., Feero W. G., Barany F., Conwit R., Hausmanowa-Petrusewicz I., Fidzianska A., Arahata K., Wessel H. B. Molecular genetic and genetic correlations in sodium channelopathies: lack of founder effect and evidence for a second gene. Am J Hum Genet. 1993 Jun;52(6):1074–1084. [PMC free article] [PubMed] [Google Scholar]
- Watkins H., Rosenzweig A., Hwang D. S., Levi T., McKenna W., Seidman C. E., Seidman J. G. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med. 1992 Apr 23;326(17):1108–1114. doi: 10.1056/NEJM199204233261703. [DOI] [PubMed] [Google Scholar]
- Watkins H., Seidman C. E., MacRae C., Seidman J. G., McKenna W. Progress in familial hypertrophic cardiomyopathy: molecular genetic analyses in the original family studied by Teare. Br Heart J. 1992 Jan;67(1):34–38. doi: 10.1136/hrt.67.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H., Thierfelder L., Hwang D. S., McKenna W., Seidman J. G., Seidman C. E. Sporadic hypertrophic cardiomyopathy due to de novo myosin mutations. J Clin Invest. 1992 Nov;90(5):1666–1671. doi: 10.1172/JCI116038. [DOI] [PMC free article] [PubMed] [Google Scholar]