Abstract
Fabry disease, an X-linked inborn error of glycosphingolipid catabolism, results from mutations in the alpha-galactosidase A (alpha-Gal A) gene at Xq22.1. To determine the nature and frequency of the molecular lesions causing the classical and milder-variant Fabry phenotypes, and for precise carrier detection in Fabry families, the alpha-Gal A transcripts or genomic sequences from unrelated Fabry hemizygotes were analyzed. In patients with the classical phenotype, 18 new mutations were identified: N34S, C56G, W162R, R227Q, R227X, D264V, D266V, S297F, D313Y, G328A, W340X, E398X, IVS2+2, IVS5 delta-2,3, 773 delta 2, 954 delta 5, 1016 delta 11, and 1123 delta 53. Unrelated asymptomatic or mildly affected patients with symptoms confined to the heart had a missense mutation, N215S, that expressed residual enzymatic activity. Related, moderately affected patients with late-onset cardiac and pulmonary manifestations had a small deletion, 1208 delta 3, that predicted the in-frame deletion of arginine 404 near the terminus of the 429 residue enzyme polypeptide. In addition, five small gene rearrangements involving exonic sequences were identified in unrelated classically affected patients. Two small deletions and one small duplication had short direct repeats at their respective breakpoint junctions and presumably resulted from slipped mispairing. A deletion occurred at a potential polymerase alpha arrest site, while the breakpoints of another deletion occurred at an inverted tetranucleotide repeat. Screening of unrelated Fabry patients with allele-specific oligonucleotides for seven mutations revealed that these were private, with the notable exception of N215S, R227Q, and R227X, which were each found in several unrelated families from different ethnic backgrounds. The CpG dinucleotide at codon 227 was the most common site of mutation, having been altered in 5% of the 148 unrelated Fabry alleles. These studies revealed that most alpha-Gal A lesions were private, that codon 227 was a mutational hot spot, and that certain mutations predicted a milder disease phenotype.
Full text
PDF

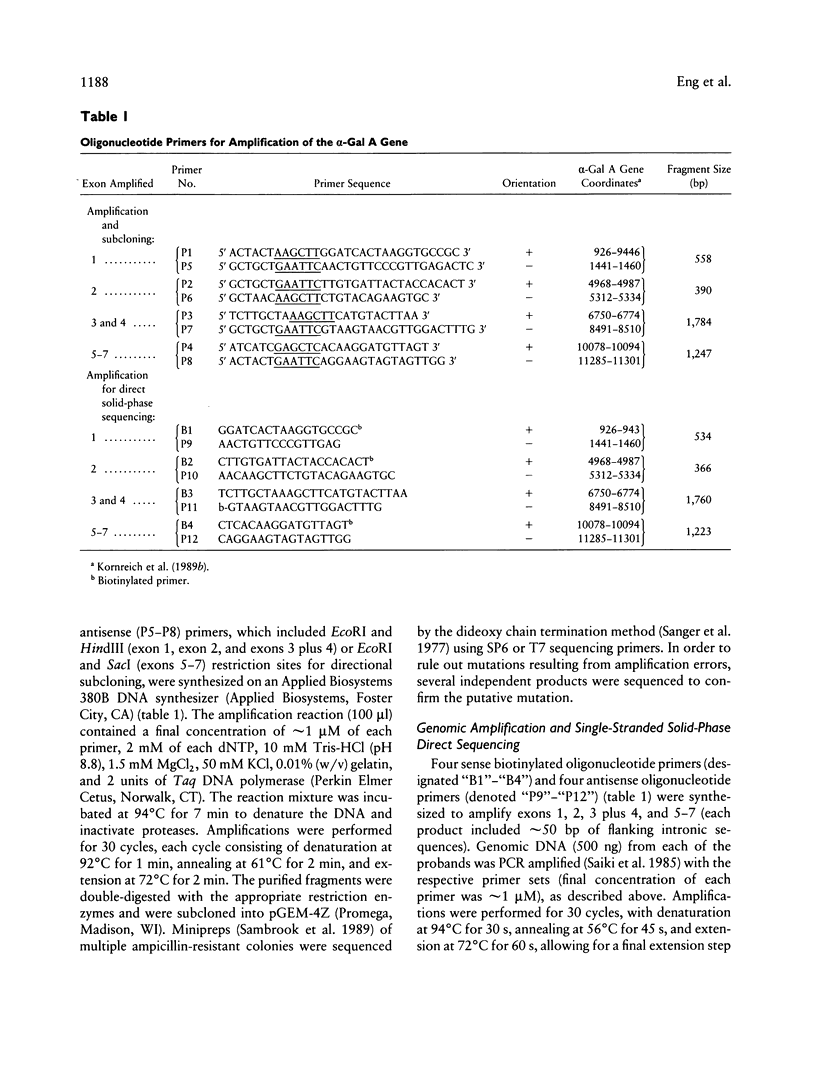
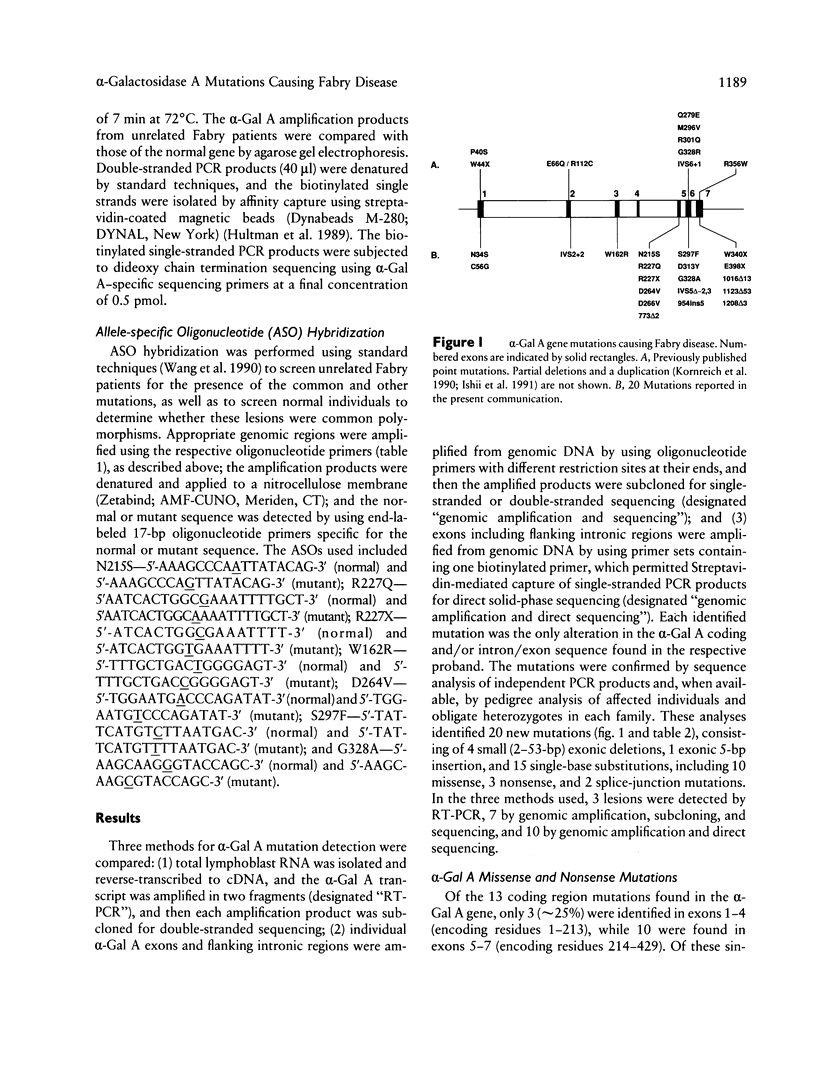




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Caskey C. T. Detection of Fabry's disease heterozygotes by hair root analysis. Clin Genet. 1978 Mar;13(3):251–258. doi: 10.1111/j.1399-0004.1978.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Bernstein H. S., Bishop D. F., Astrin K. H., Kornreich R., Eng C. M., Sakuraba H., Desnick R. J. Fabry disease: six gene rearrangements and an exonic point mutation in the alpha-galactosidase gene. J Clin Invest. 1989 Apr;83(4):1390–1399. doi: 10.1172/JCI114027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Calhoun D. H., Bernstein H. S., Hantzopoulos P., Quinn M., Desnick R. J. Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4859–4863. doi: 10.1073/pnas.83.13.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Kornreich R., Desnick R. J. Structural organization of the human alpha-galactosidase A gene: further evidence for the absence of a 3' untranslated region. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3903–3907. doi: 10.1073/pnas.85.11.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Cho H. I., Sommer S. S. Hemophilia B in a male with a four-base insertion that arose in the germline of his mother. Nucleic Acids Res. 1989 Dec 11;17(23):10139–10139. doi: 10.1093/nar/17.23.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Krawczak M. Mechanisms of insertional mutagenesis in human genes causing genetic disease. Hum Genet. 1991 Aug;87(4):409–415. doi: 10.1007/BF00197158. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Allen K. Y., Desnick S. J., Raman M. K., Bernlohr R. W., Krivit W. Fabry's disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab Clin Med. 1973 Feb;81(2):157–171. [PubMed] [Google Scholar]
- Dietz H. C., Valle D., Francomano C. A., Kendzior R. J., Jr, Pyeritz R. E., Cutting G. R. The skipping of constitutive exons in vivo induced by nonsense mutations. Science. 1993 Jan 29;259(5095):680–683. doi: 10.1126/science.8430317. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Elleder M., Bradová V., Smíd F., Budesínský M., Harzer K., Kustermann-Kuhn B., Ledvinová J., Belohlávek, Král V., Dorazilová V. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry's disease. Report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol Anat Histopathol. 1990;417(5):449–455. doi: 10.1007/BF01606034. [DOI] [PubMed] [Google Scholar]
- Howells D. W., Forrest S. M., Dahl H. H., Cotton R. G. Insertion of an extra codon for threonine is a cause of dihydropteridine reductase deficiency. Am J Hum Genet. 1990 Aug;47(2):279–285. [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou Y. A., Bishop D. F., Desnick R. J. Overexpression of human alpha-galactosidase A results in its intracellular aggregation, crystallization in lysosomes, and selective secretion. J Cell Biol. 1992 Dec;119(5):1137–1150. doi: 10.1083/jcb.119.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
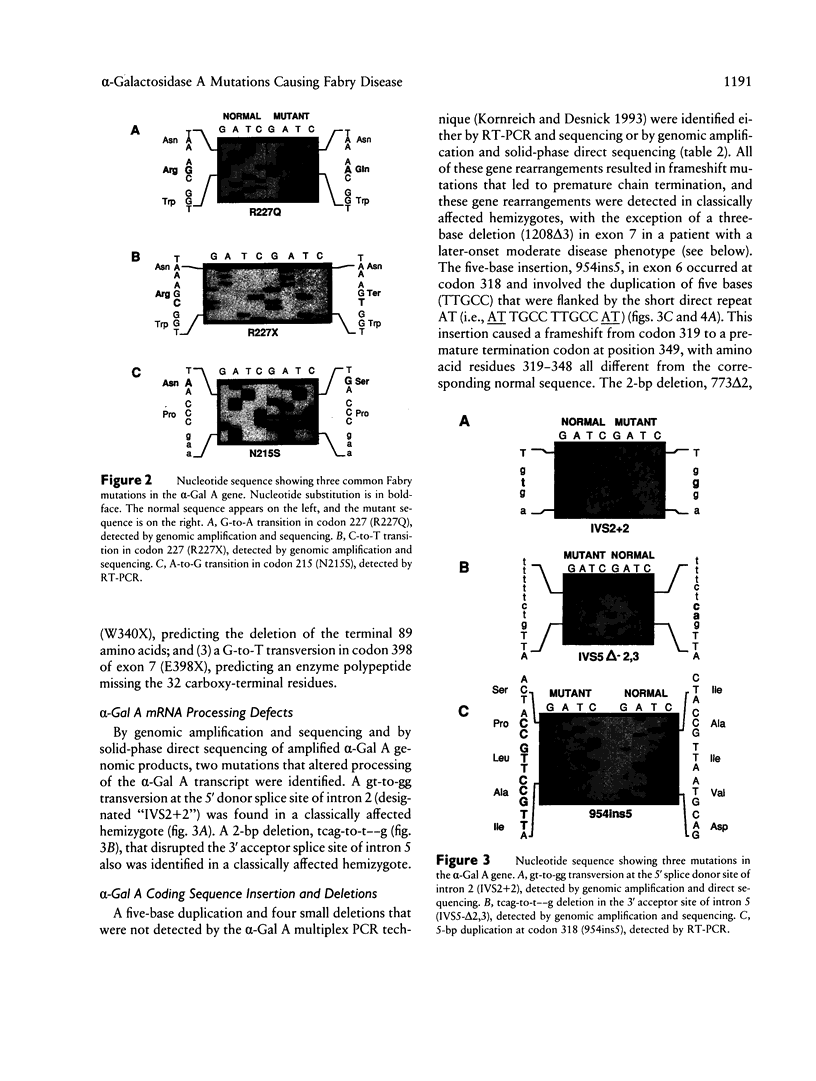
- Ishii S., Sakuraba H., Shimmoto M., Minamikawa-Tachino R., Suzuki T., Suzuki Y. Fabry disease: detection of 13-bp deletion in alpha-galactosidase A gene and its application to gene diagnosis of heterozygotes. Ann Neurol. 1991 May;29(5):560–564. doi: 10.1002/ana.410290517. [DOI] [PubMed] [Google Scholar]
- Ishii S., Sakuraba H., Suzuki Y. Point mutations in the upstream region of the alpha-galactosidase A gene exon 6 in an atypical variant of Fabry disease. Hum Genet. 1992 Apr;89(1):29–32. doi: 10.1007/BF00207037. [DOI] [PubMed] [Google Scholar]
- Koide T., Ishiura M., Iwai K., Inoue M., Kaneda Y., Okada Y., Uchida T. A case of Fabry's disease in a patient with no alpha-galactosidase A activity caused by a single amino acid substitution of Pro-40 by Ser. FEBS Lett. 1990 Jan 1;259(2):353–356. doi: 10.1016/0014-5793(90)80046-l. [DOI] [PubMed] [Google Scholar]
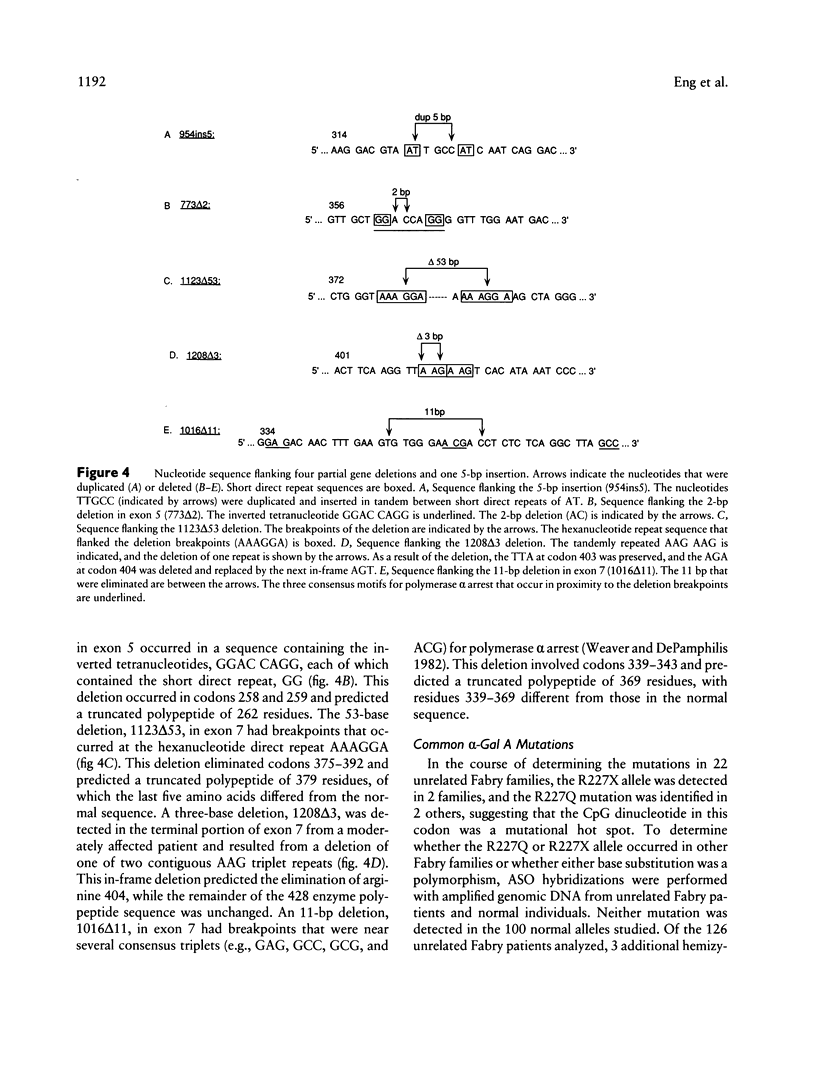
- Kornreich R., Bishop D. F., Desnick R. J. The gene encoding alpha-galactosidase A and gene rearrangements causing Fabry disease. Trans Assoc Am Physicians. 1989;102:30–43. [PubMed] [Google Scholar]
- Kornreich R., Desnick R. J., Bishop D. F. Nucleotide sequence of the human alpha-galactosidase A gene. Nucleic Acids Res. 1989 Apr 25;17(8):3301–3302. doi: 10.1093/nar/17.8.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich R., Desnick R. J. Fabry disease: detection of gene rearrangements in the human alpha-galactosidase A gene by multiplex PCR amplification. Hum Mutat. 1993;2(2):108–111. doi: 10.1002/humu.1380020208. [DOI] [PubMed] [Google Scholar]
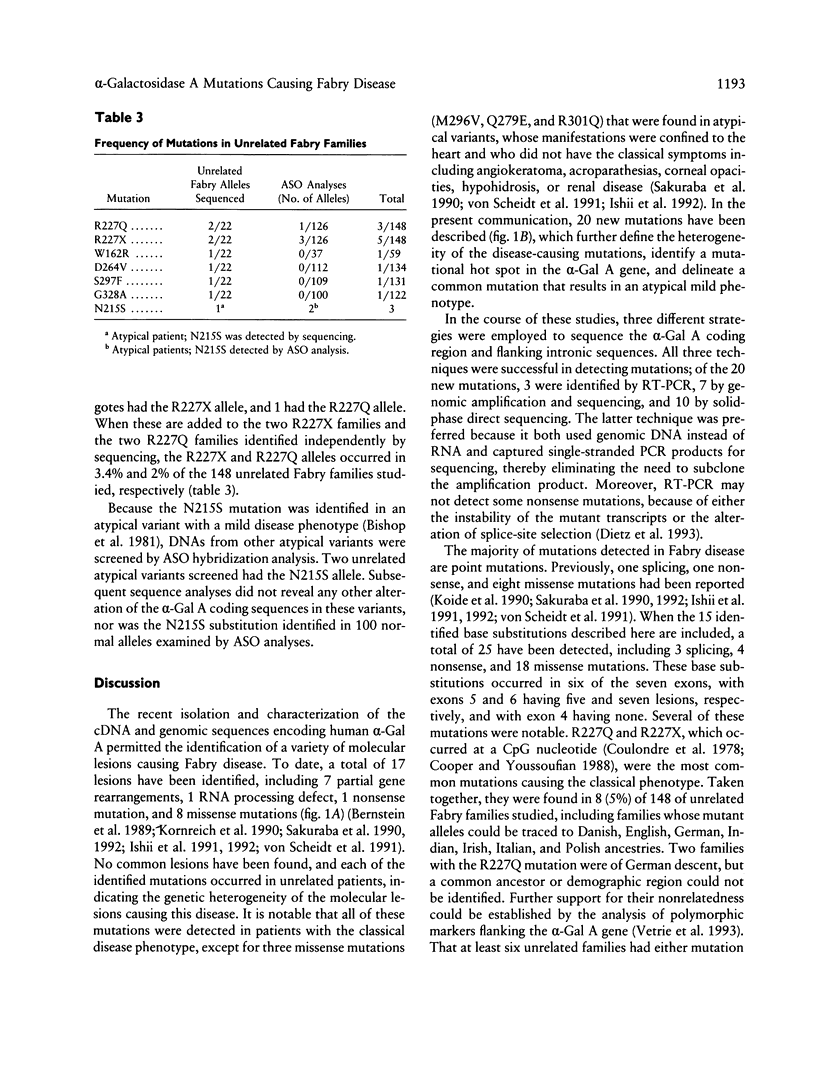
- Krawczak M., Cooper D. N. Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet. 1991 Mar;86(5):425–441. doi: 10.1007/BF00194629. [DOI] [PubMed] [Google Scholar]
- Romeo G., Migeon B. R. Genetic inactivation of the alpha-galactosidase locus in carriers of Fabry's disease. Science. 1970 Oct 9;170(3954):180–181. doi: 10.1126/science.170.3954.180. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sakuraba H., Eng C. M., Desnick R. J., Bishop D. F. Invariant exon skipping in the human alpha-galactosidase A pre-mRNA: Ag+1 to t substitution in a 5'-splice site causing Fabry disease. Genomics. 1992 Apr;12(4):643–650. doi: 10.1016/0888-7543(92)90288-4. [DOI] [PubMed] [Google Scholar]
- Sakuraba H., Oshima A., Fukuhara Y., Shimmoto M., Nagao Y., Bishop D. F., Desnick R. J., Suzuki Y. Identification of point mutations in the alpha-galactosidase A gene in classical and atypical hemizygotes with Fabry disease. Am J Hum Genet. 1990 Nov;47(5):784–789. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrie D., Bobrow M., Harris A. Construction of a 5.2-megabase physical map of the human X chromosome at Xq22 using pulsed-field gel electrophoresis and yeast artificial chromosomes. Genomics. 1993 Mar;15(3):631–642. doi: 10.1006/geno.1993.1118. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Schindler D., Desnick R. Schindler disease: the molecular lesion in the alpha-N-acetylgalactosaminidase gene that causes an infantile neuroaxonal dystrophy. J Clin Invest. 1990 Nov;86(5):1752–1756. doi: 10.1172/JCI114901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. T., DePamphilis M. L. Specific sequences in native DNA that arrest synthesis by DNA polymerase alpha. J Biol Chem. 1982 Feb 25;257(4):2075–2086. [PubMed] [Google Scholar]
- von Scheidt W., Eng C. M., Fitzmaurice T. F., Erdmann E., Hübner G., Olsen E. G., Christomanou H., Kandolf R., Bishop D. F., Desnick R. J. An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med. 1991 Feb 7;324(6):395–399. doi: 10.1056/NEJM199102073240607. [DOI] [PubMed] [Google Scholar]