Abstract
The profound influence that the genetic makeup of the host has on resistance to malaria infection has been established in numerous animal studies. This genetic heterogeneity is one of the main causes of the difficulties in developing an effective malaria vaccine. Segregation analysis is the first step in identifying the nature of genetic factors involved in the expression of human complex diseases, as infectious diseases. To assess the role of host genes in human malaria, we performed segregation analysis of blood parasite densities in 42 Cameroonian families by using both the unified mixed model and the class D regressive model of analysis. The results provide clear evidence for the presence of a recessive major gene controlling the degree of infection in human malaria. Parameter estimates show a frequency of .44-.48 for the deleterious allele, indicating that about 21% of the population is predisposed to high levels of infection.
Full text
PDF
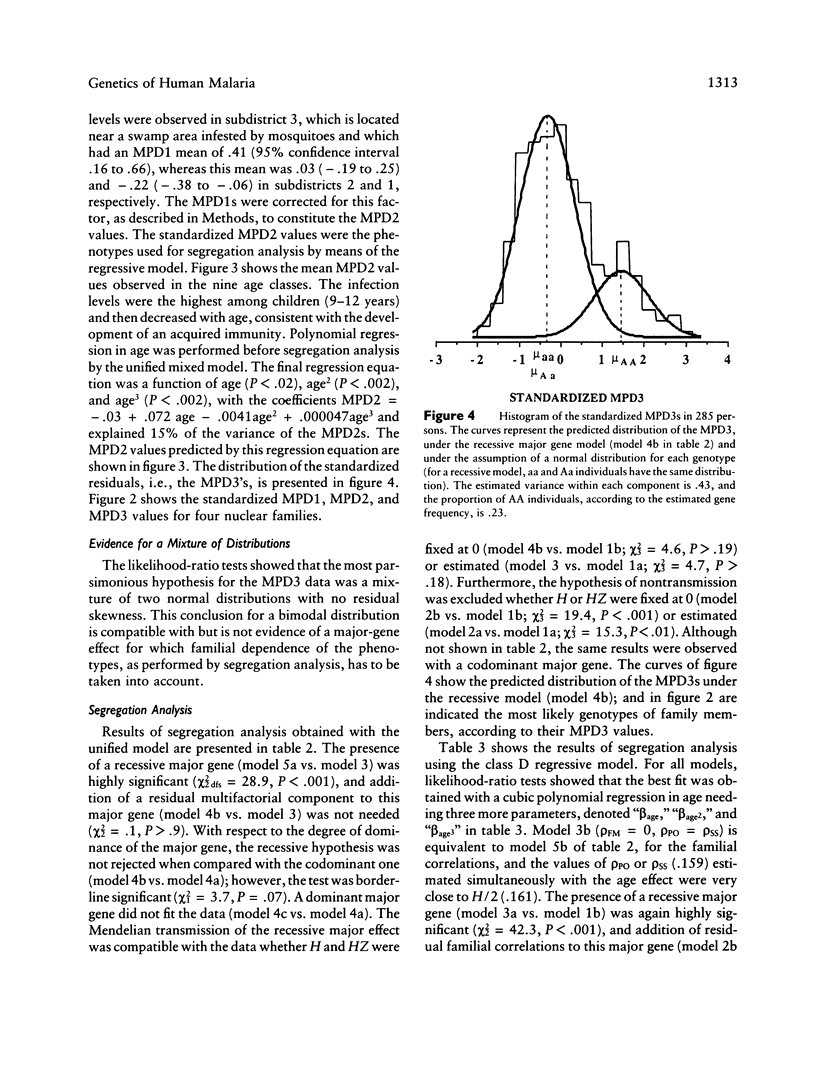
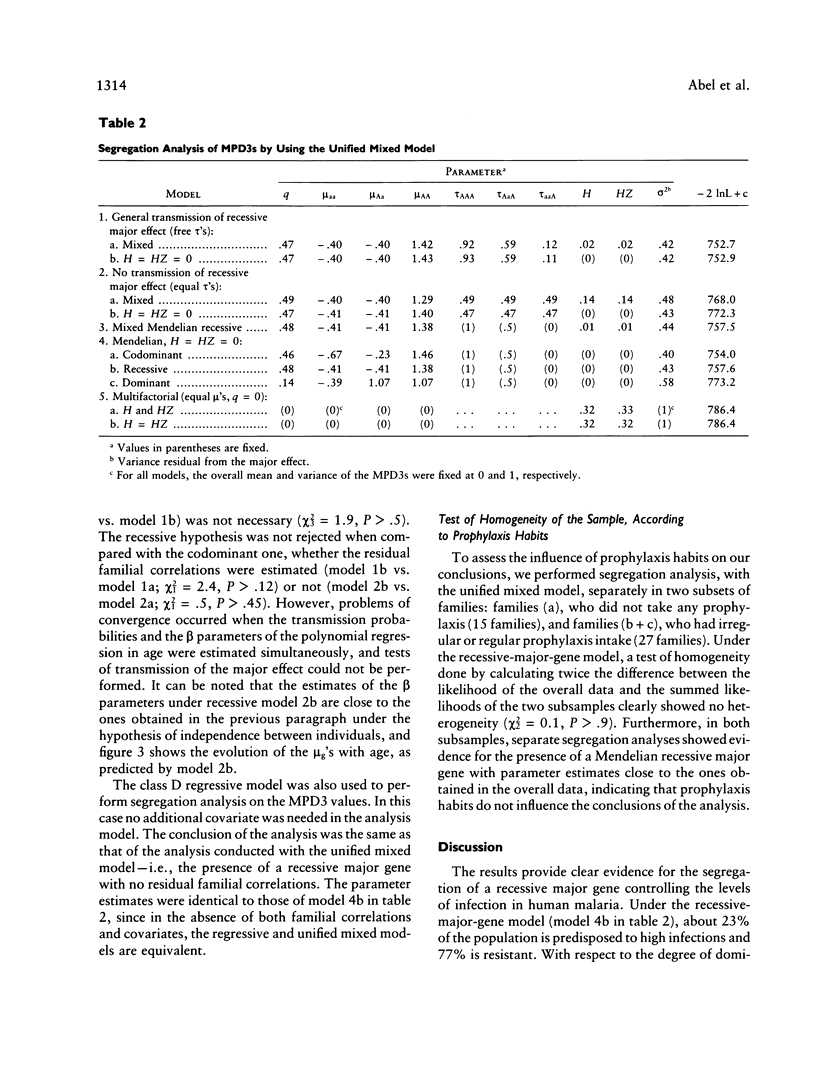
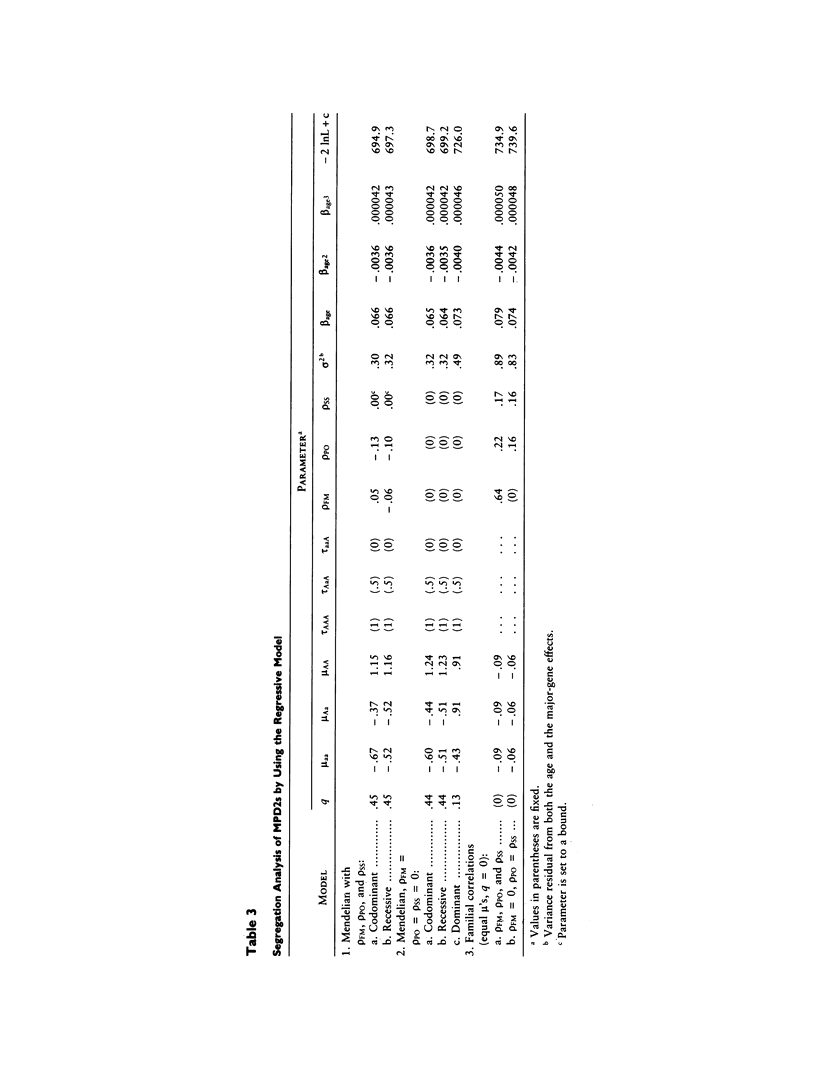


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel L., Demenais F. Detection of major genes for susceptibility to leprosy and its subtypes in a Caribbean island: Desirade island. Am J Hum Genet. 1988 Feb;42(2):256–266. [PMC free article] [PubMed] [Google Scholar]
- Abel L., Demenais F., Prata A., Souza A. E., Dessein A. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet. 1991 May;48(5):959–970. [PMC free article] [PubMed] [Google Scholar]
- Bonney G. E. On the statistical determination of major gene mechanisms in continuous human traits: regressive models. Am J Med Genet. 1984 Aug;18(4):731–749. doi: 10.1002/ajmg.1320180420. [DOI] [PubMed] [Google Scholar]
- Demenais F. M., Bonney G. E. Equivalence of the mixed and regressive models for genetic analysis. I. Continuous traits. Genet Epidemiol. 1989;6(5):597–617. doi: 10.1002/gepi.1370060505. [DOI] [PubMed] [Google Scholar]
- Demenais F., Lathrop M., Lalouel J. M. Robustness and power of the unified model in the analysis of quantitative measurements. Am J Hum Genet. 1986 Feb;38(2):228–234. [PMC free article] [PubMed] [Google Scholar]
- Gazin P., Louis J. P., Mulder L., Eberle F., Jambou R., Moyroud, Hengy C. Evaluation par test simplifié in vivo de la chimiosensibilité du Plasmodium falciparum à la chloroquine et à l'amodiaquine dans le Sud du Cameroun. Med Trop (Mars) 1990 Jan-Mar;50(1):27–31. [PubMed] [Google Scholar]
- Good M. F., Miller L. H., Kumar S., Quakyi I. A., Keister D., Adams J. H., Moss B., Berzofsky J. A., Carter R. Limited immunological recognition of critical malaria vaccine candidate antigens. Science. 1988 Oct 28;242(4878):574–577. doi: 10.1126/science.2902690. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Lalouel J. M., Morton N. E. Complex segregation analysis with pointers. Hum Hered. 1981;31(5):312–321. doi: 10.1159/000153231. [DOI] [PubMed] [Google Scholar]
- Lalouel J. M., Rao D. C., Morton N. E., Elston R. C. A unified model for complex segregation analysis. Am J Hum Genet. 1983 Sep;35(5):816–826. [PMC free article] [PubMed] [Google Scholar]
- Londoño J. A., Gras-Masse H., Dubeaux C., Tartar A., Druilhe P. Secondary structure and immunogenicity of hybrid synthetic peptides derived from two Plasmodium falciparum pre-erythrocytic antigens. J Immunol. 1990 Sep 1;145(5):1557–1563. [PubMed] [Google Scholar]
- Maclean C. J., Morton N. E., Elston R. C., Yee S. Skewness in commingled distributions. Biometrics. 1976 Sep;32(3):695–699. [PubMed] [Google Scholar]
- Miller L. H., Howard R. J., Carter R., Good M. F., Nussenzweig V., Nussenzweig R. S. Research toward malaria vaccines. Science. 1986 Dec 12;234(4782):1349–1356. doi: 10.1126/science.2431481. [DOI] [PubMed] [Google Scholar]
- Rice T., Bouchard C., Borecki I. B., Rao D. C. Commingling and segregation analysis of blood pressure in a French-Canadian population. Am J Hum Genet. 1990 Jan;46(1):37–44. [PMC free article] [PubMed] [Google Scholar]
- Sayles P. C., Wassom D. L. Immunoregulation in murine malaria. Susceptibility of inbred mice to infection with Plasmodium yoelii depends on the dynamic interplay of host and parasite genes. J Immunol. 1988 Jul 1;141(1):241–248. [PubMed] [Google Scholar]
- Schurr E., Skamene E., Forget A., Gros P. Linkage analysis of the Bcg gene on mouse chromosome 1. Identification of a tightly linked marker. J Immunol. 1989 Jun 15;142(12):4507–4513. [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Skamene E. Murine malaria: resistance of AXB/BXA recombinant inbred mice to Plasmodium chabaudi. Infect Immun. 1985 Feb;47(2):452–456. doi: 10.1128/iai.47.2.452-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trape J. F. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans R Soc Trop Med Hyg. 1985;79(2):181–184. doi: 10.1016/0035-9203(85)90329-3. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J. Common genetic disorders of the red cell and the 'malaria hypothesis'. Ann Trop Med Parasitol. 1987 Oct;81(5):539–548. doi: 10.1080/00034983.1987.11812155. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Good M. F., Hollingdale M. R., Miller L. H., Berzofsky J. A. Genetic control of immunity to Plasmodium yoelii sporozoites. J Immunol. 1989 Dec 15;143(12):4263–4266. [PubMed] [Google Scholar]
- Wunderlich F., Mossmann H., Helwig M., Schillinger G. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect Immun. 1988 Sep;56(9):2400–2406. doi: 10.1128/iai.56.9.2400-2406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



