Abstract
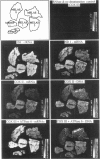
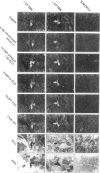
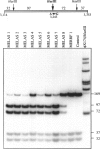
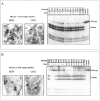
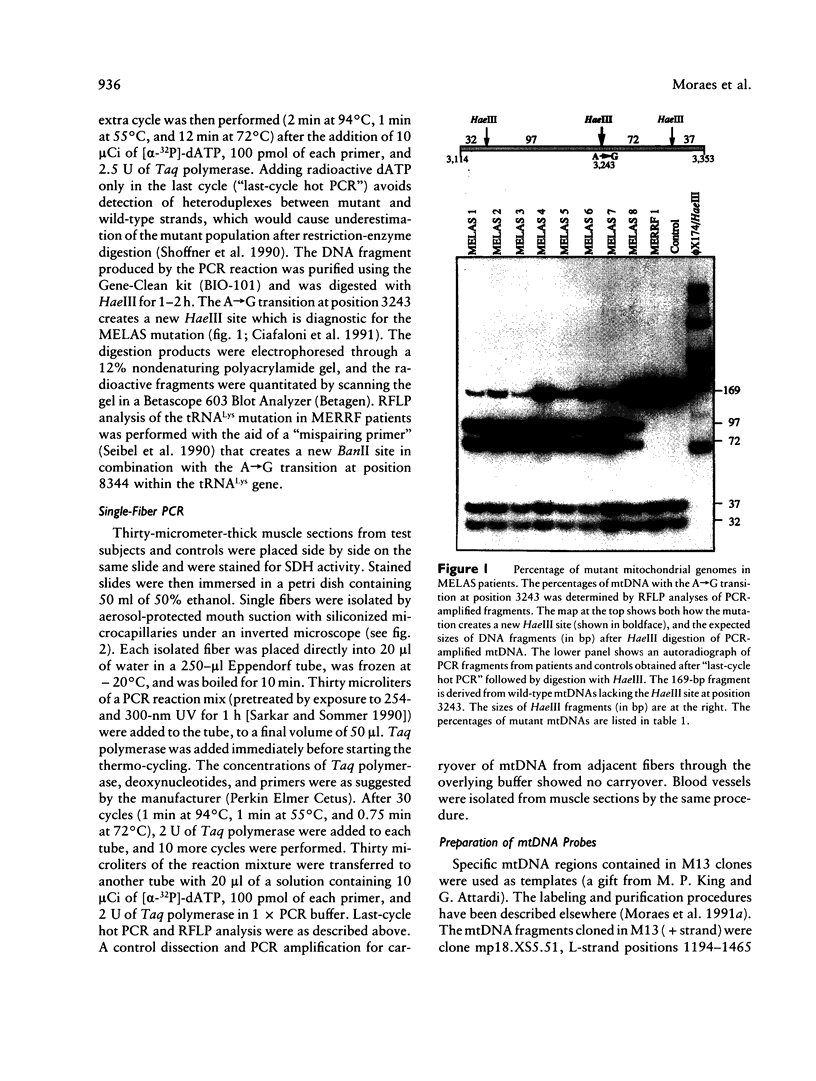
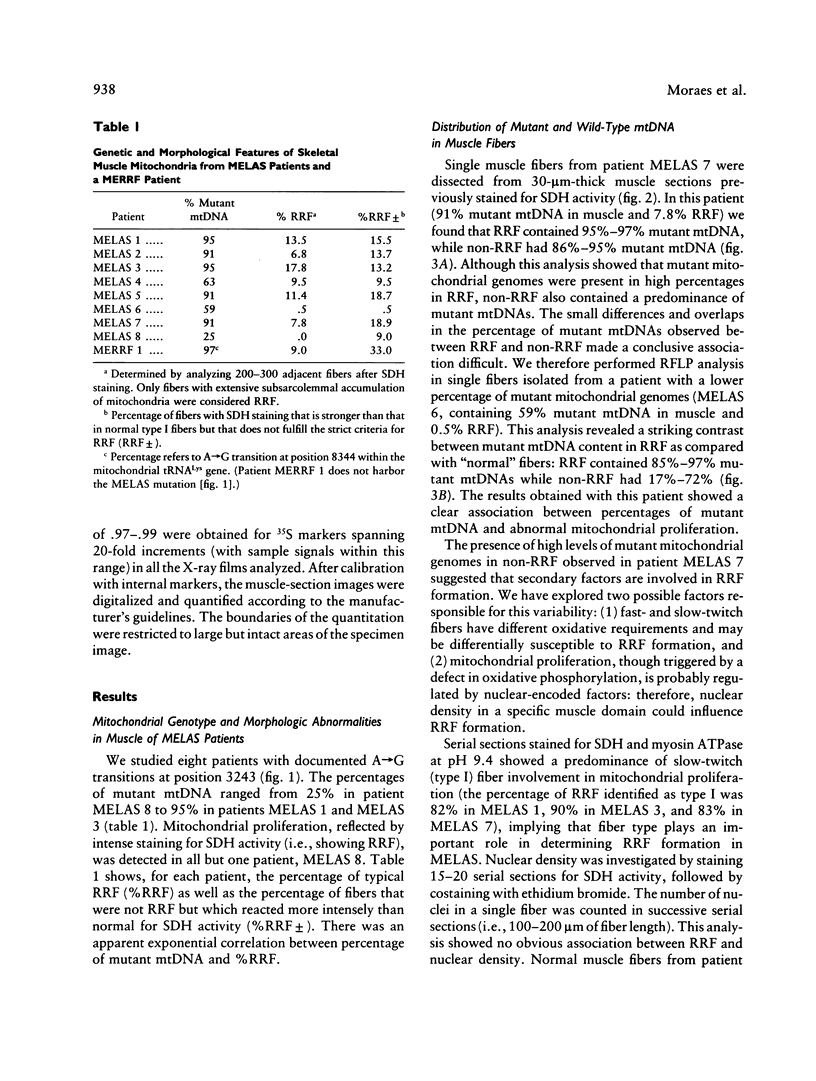
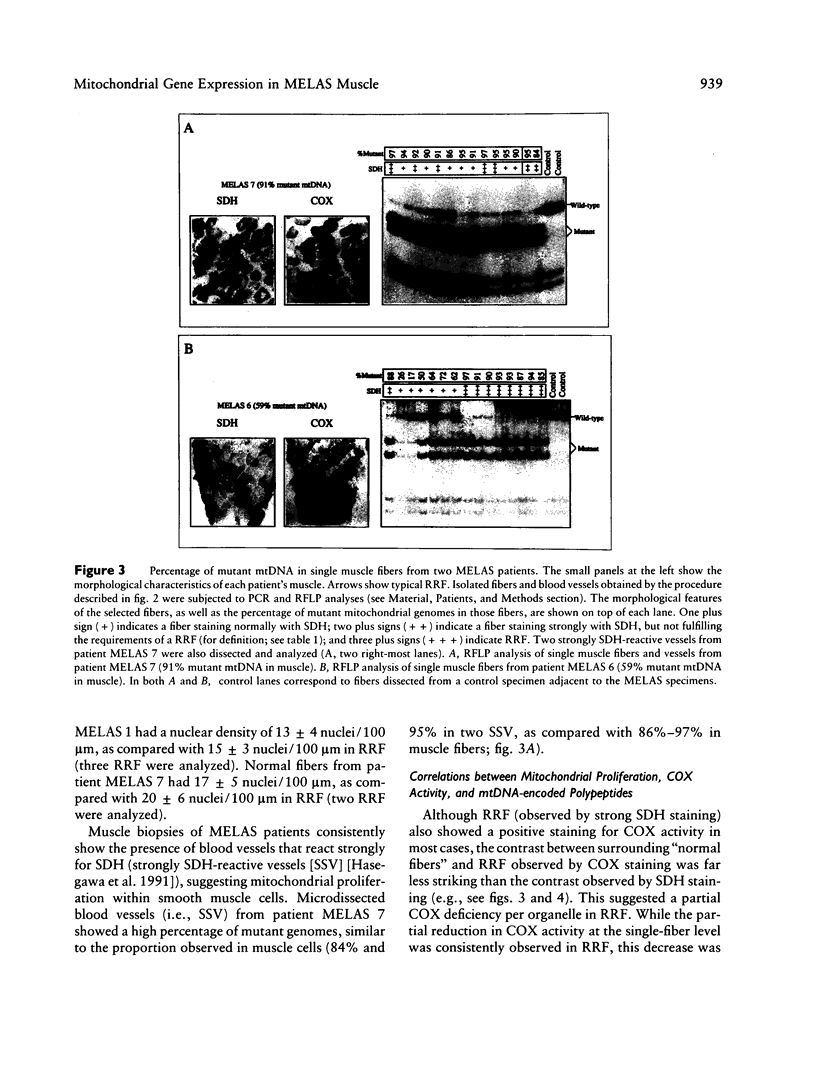
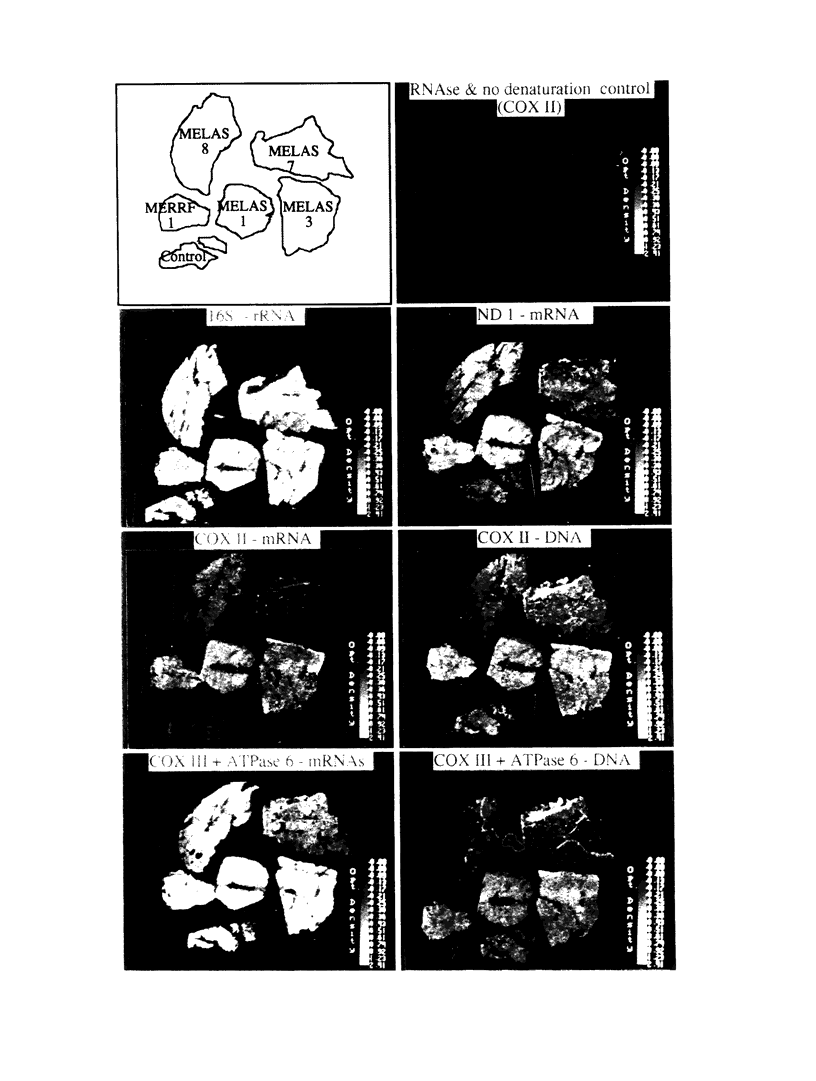
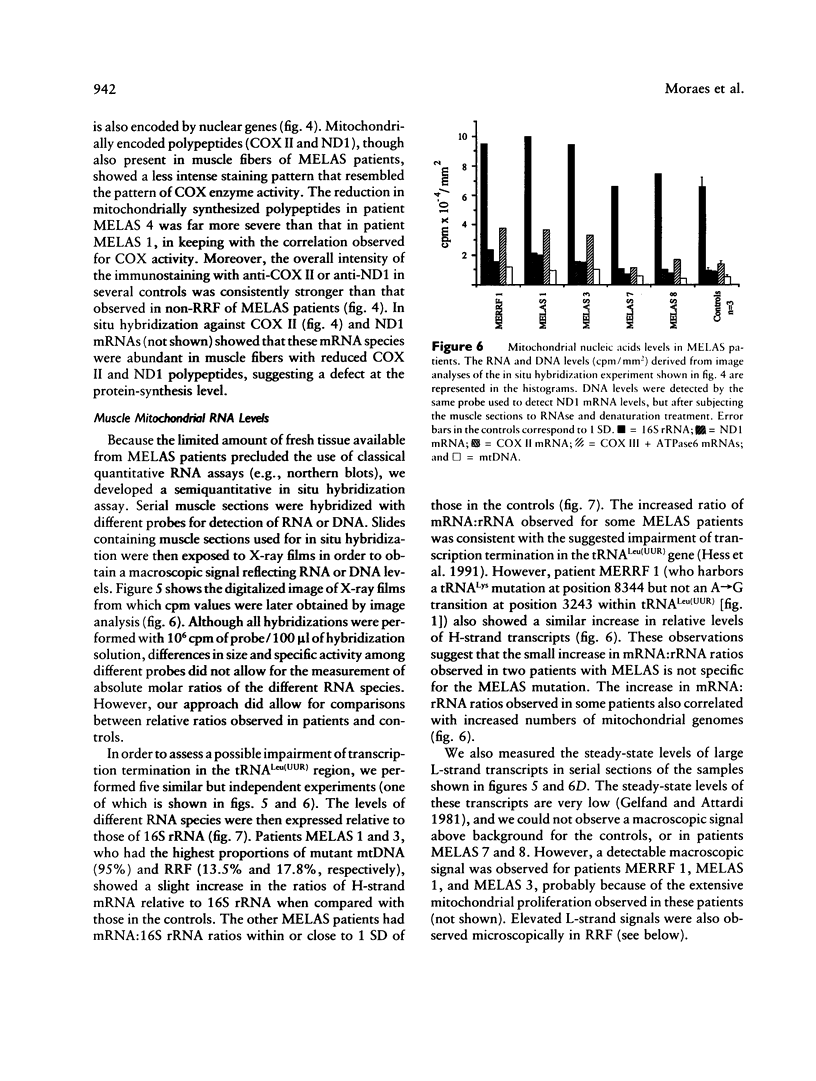
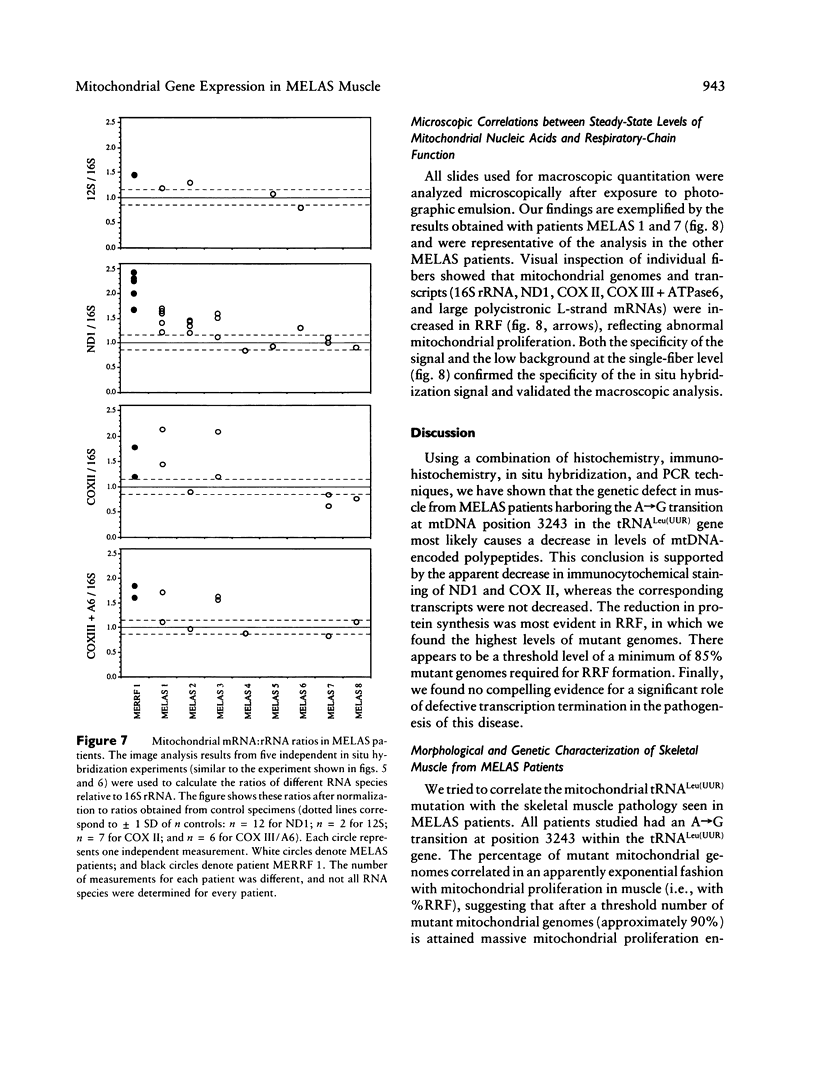
Mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes (MELAS) has recently been associated with an A----G transition at position 3243 within the mitochondrial tRNA(Leu(UUR)) gene. Besides altering the tRNA(Leu(UUR)) sequence, this point mutation lies within a DNA segment responsible for transcription termination of the rRNA genes. We have studied the distribution and expression of mutant mtDNAs in muscle biopsies from MELAS patients. Histochemical, immunohistochemical, and single-fiber PCR analysis showed that ragged-red fibers (RRF) are associated both with high levels of mutant mitochondrial genomes (greater than 85% mutant mtDNA) and with a partial cytochrome c oxidase deficiency. By quantitative in situ hybridization, the steady-state ratios of mRNAs:rRNAs were found to be similar to controls in six of eight patients studied. In two other patients the relative levels of heavy-strand mRNAs were slightly increased, but a patient with myoclonic epilepsy and RRF also exhibited a similar increase. These results directly correlate the A----G transition at mtDNA position 3243 with muscle mitochondrial proliferation, partial respiratory-chain impairment, decreased mitochondrially synthesized protein content, and no specific alterations in mitochondrial ratios of mRNAs:rRNAs.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc Natl Acad Sci U S A. 1985 Jan;82(2):351–355. doi: 10.1073/pnas.82.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Clayton D. A. In vitro transcription of human mitochondrial DNA: accurate termination requires a region of DNA sequence that can function bidirectionally. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6277–6281. doi: 10.1073/pnas.83.17.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciafaloni E., Ricci E., Servidei S., Shanske S., Silvestri G., Manfredi G., Schon E. A., DiMauro S. Widespread tissue distribution of a tRNALeu(UUR) mutation in the mitochondrial DNA of a patient with MELAS syndrome. Neurology. 1991 Oct;41(10):1663–1664. doi: 10.1212/wnl.41.10.1663. [DOI] [PubMed] [Google Scholar]
- Ciafaloni E., Ricci E., Shanske S., Moraes C. T., Silvestri G., Hirano M., Simonetti S., Angelini C., Donati M. A., Garcia C. MELAS: clinical features, biochemistry, and molecular genetics. Ann Neurol. 1992 Apr;31(4):391–398. doi: 10.1002/ana.410310408. [DOI] [PubMed] [Google Scholar]
- Goto Y., Koga Y., Horai S., Nonaka I. Chronic progressive external ophthalmoplegia: a correlative study of mitochondrial DNA deletions and their phenotypic expression in muscle biopsies. J Neurol Sci. 1990 Dec;100(1-2):63–69. doi: 10.1016/0022-510x(90)90014-e. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A new mtDNA mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Biochim Biophys Acta. 1991 Oct 21;1097(3):238–240. doi: 10.1016/0925-4439(91)90042-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Matsuoka T., Goto Y., Nonaka I. Strongly succinate dehydrogenase-reactive blood vessels in muscles from patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. Ann Neurol. 1991 Jun;29(6):601–605. doi: 10.1002/ana.410290606. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Parisi M. A., Bennett J. L., Clayton D. A. Impairment of mitochondrial transcription termination by a point mutation associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1991 May 16;351(6323):236–239. doi: 10.1038/351236a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Cooper J. M., Schapira A. H., Toscano A., Clark J. B., Morgan-Hughes J. A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989 Dec;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Xu M., McCullough D. A. Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. Am J Hum Genet. 1991 May;48(5):935–942. [PMC free article] [PubMed] [Google Scholar]
- Huoponen K., Vilkki J., Aula P., Nikoskelainen E. K., Savontaus M. L. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1991 Jun;48(6):1147–1153. [PMC free article] [PubMed] [Google Scholar]
- Karpati G., Carpenter S., Larbrisseau A., Lafontaine R. The Kearns-Shy syndrome. A multisystem disease with mitochondrial abnormality demonstrated in skeletal muscle and skin. J Neurol Sci. 1973 Jun;19(2):133–151. doi: 10.1016/0022-510x(73)90158-5. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Momoi T., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun. 1990 Dec 31;173(3):816–822. doi: 10.1016/s0006-291x(05)80860-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Shimoizumi H., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. Respiration-deficient cells are caused by a single point mutation in the mitochondrial tRNA-Leu (UUR) gene in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS). Am J Hum Genet. 1991 Sep;49(3):590–599. [PMC free article] [PubMed] [Google Scholar]
- Koga Y., Nonaka I., Kobayashi M., Tojyo M., Nihei K. Findings in muscle in complex I (NADH coenzyme Q reductase) deficiency. Ann Neurol. 1988 Dec;24(6):749–756. doi: 10.1002/ana.410240609. [DOI] [PubMed] [Google Scholar]
- Kruse B., Narasimhan N., Attardi G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989 Jul 28;58(2):391–397. doi: 10.1016/0092-8674(89)90853-2. [DOI] [PubMed] [Google Scholar]
- Lombes A., Mendell J. R., Nakase H., Barohn R. J., Bonilla E., Zeviani M., Yates A. J., Omerza J., Gales T. L., Nakahara K. Myoclonic epilepsy and ragged-red fibers with cytochrome oxidase deficiency: neuropathology, biochemistry, and molecular genetics. Ann Neurol. 1989 Jul;26(1):20–33. doi: 10.1002/ana.410260104. [DOI] [PubMed] [Google Scholar]
- Mita S., Schmidt B., Schon E. A., DiMauro S., Bonilla E. Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9509–9513. doi: 10.1073/pnas.86.23.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Andreetta F., Bonilla E., Shanske S., DiMauro S., Schon E. A. Replication-competent human mitochondrial DNA lacking the heavy-strand promoter region. Mol Cell Biol. 1991 Mar;11(3):1631–1637. doi: 10.1128/mcb.11.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Nelson I., Degoul F., Obermaier-Kusser B., Romero N., Borrone C., Marsac C., Vayssiere J. L., Gerbitz K., Fardeau M., Ponsot G. Mapping of heteroplasmic mitochondrial DNA deletions in Kearns-Sayre syndrome. Nucleic Acids Res. 1989 Oct 25;17(20):8117–8124. doi: 10.1093/nar/17.20.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W., Engel W. K., Walsh G. O., Einaugler R. Oculocraniosomatic neuromuscular disease with "ragged-red" fibers. Arch Neurol. 1972 Mar;26(3):193–211. doi: 10.1001/archneur.1972.00490090019001. [DOI] [PubMed] [Google Scholar]
- Pavlakis S. G., Phillips P. C., DiMauro S., De Vivo D. C., Rowland L. P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984 Oct;16(4):481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- Romero N. B., Lestienne P., Marsac C., Paturneau-Jouas M., Nelson I., François D., Eymard B., Fardeau M. Immunocytological and histochemical correlation in Kearns-Sayre syndrome with mtDNA deletion and partial cytochrome c oxidase deficiency in skeletal muscle. J Neurol Sci. 1989 Nov;93(2-3):297–309. doi: 10.1016/0022-510x(89)90199-8. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Seibel P., Degoul F., Romero N., Marsac C., Kadenbach B. Identification of point mutations by mispairing PCR as exemplified in MERRF disease. Biochem Biophys Res Commun. 1990 Dec 14;173(2):561–565. doi: 10.1016/s0006-291x(05)80071-3. [DOI] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J. M., 4th, Wallace D. C. Oxidative phosphorylation diseases. Disorders of two genomes. Adv Hum Genet. 1990;19:267–330. [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Karpati G., Hastings K. E. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell. 1990 Jul 13;62(1):43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- Sudoyo H., Marzuki S., Trounce I., Byrne E. Antimitochondrial autoantibodies of primary biliary cirrhosis as a novel probe in the study of 2-oxo acid dehydrogenases in patients with mitochondrial myopathies. J Neurol Sci. 1990 Sep;98(2-3):185–193. doi: 10.1016/0022-510x(90)90259-p. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ino H., Ohno K., Ohbayashi T., Ikebe S., Sano T., Ichiki T., Kobayashi M., Wada Y., Ozawa T. Mitochondrial DNA mutations in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Biochem Biophys Res Commun. 1991 Jan 31;174(2):861–868. doi: 10.1016/0006-291x(91)91497-z. [DOI] [PubMed] [Google Scholar]
- Tritschler H. J., Bonilla E., Lombes A., Andreetta F., Servidei S., Schneyder B., Miranda A. F., Schon E. A., Kadenbach B., DiMauro S. Differential diagnosis of fatal and benign cytochrome c oxidase-deficient myopathies of infancy: an immunohistochemical approach. Neurology. 1991 Feb;41(2 ):300–305. doi: 10.1212/wnl.41.2_part_1.300. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Tanno Y., Horai S., Ozawa T., Miyatake T., Tsuji S. A common mitochondrial DNA mutation in the t-RNA(Lys) of patients with myoclonus epilepsy associated with ragged-red fibers. Biochem Int. 1990 Aug;21(5):789–796. [PubMed] [Google Scholar]
- Zeviani M., Gellera C., Antozzi C., Rimoldi M., Morandi L., Villani F., Tiranti V., DiDonato S. Maternally inherited myopathy and cardiomyopathy: association with mutation in mitochondrial DNA tRNA(Leu)(UUR). Lancet. 1991 Jul 20;338(8760):143–147. doi: 10.1016/0140-6736(91)90136-d. [DOI] [PubMed] [Google Scholar]