Abstract
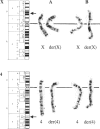
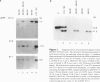
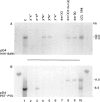
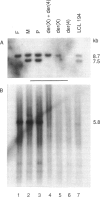
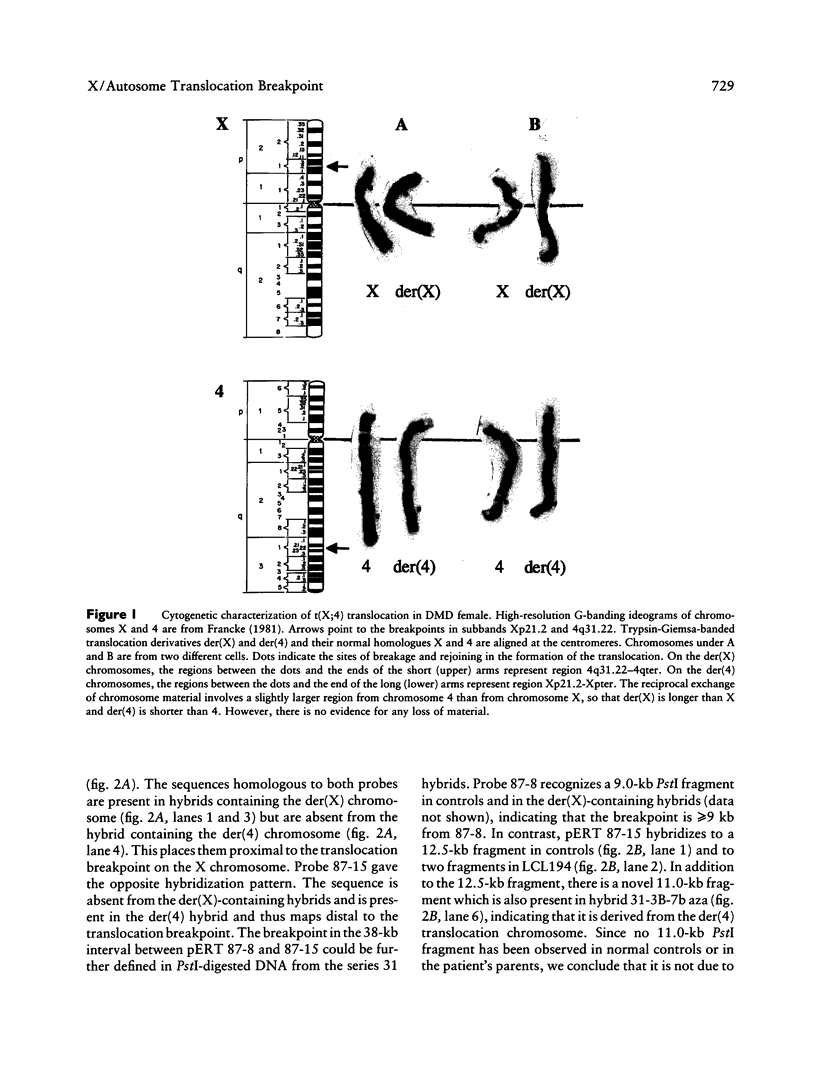
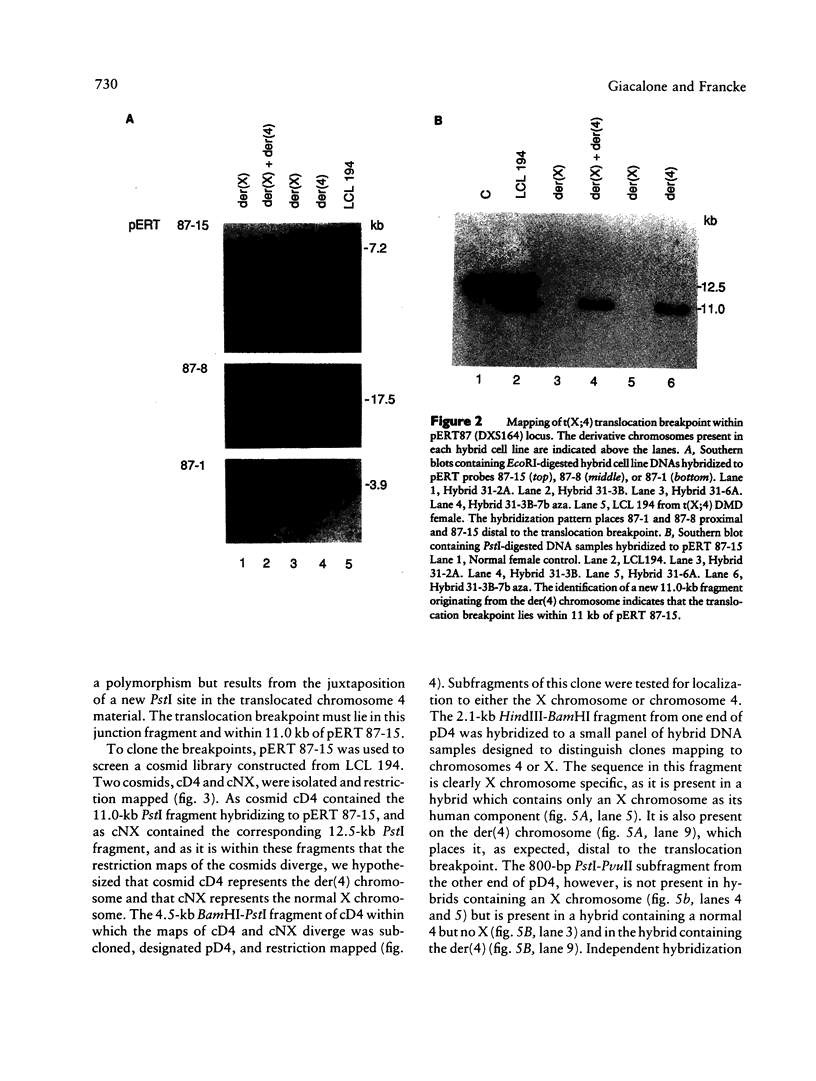
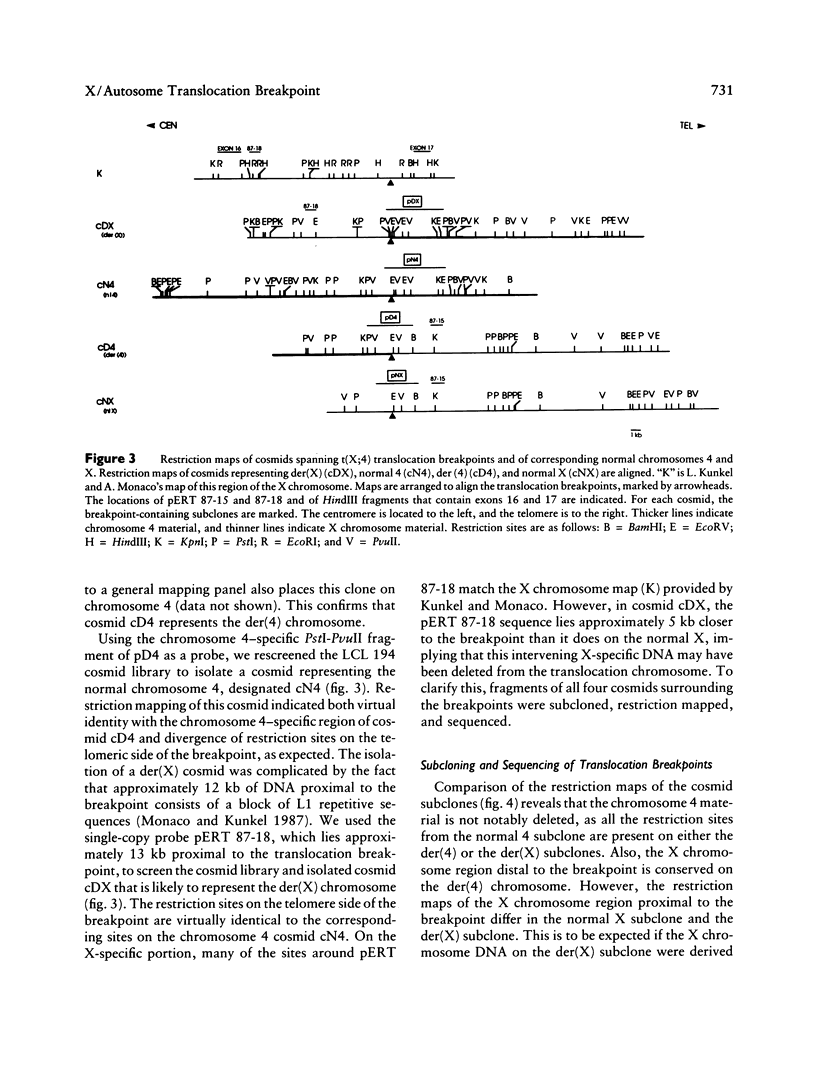
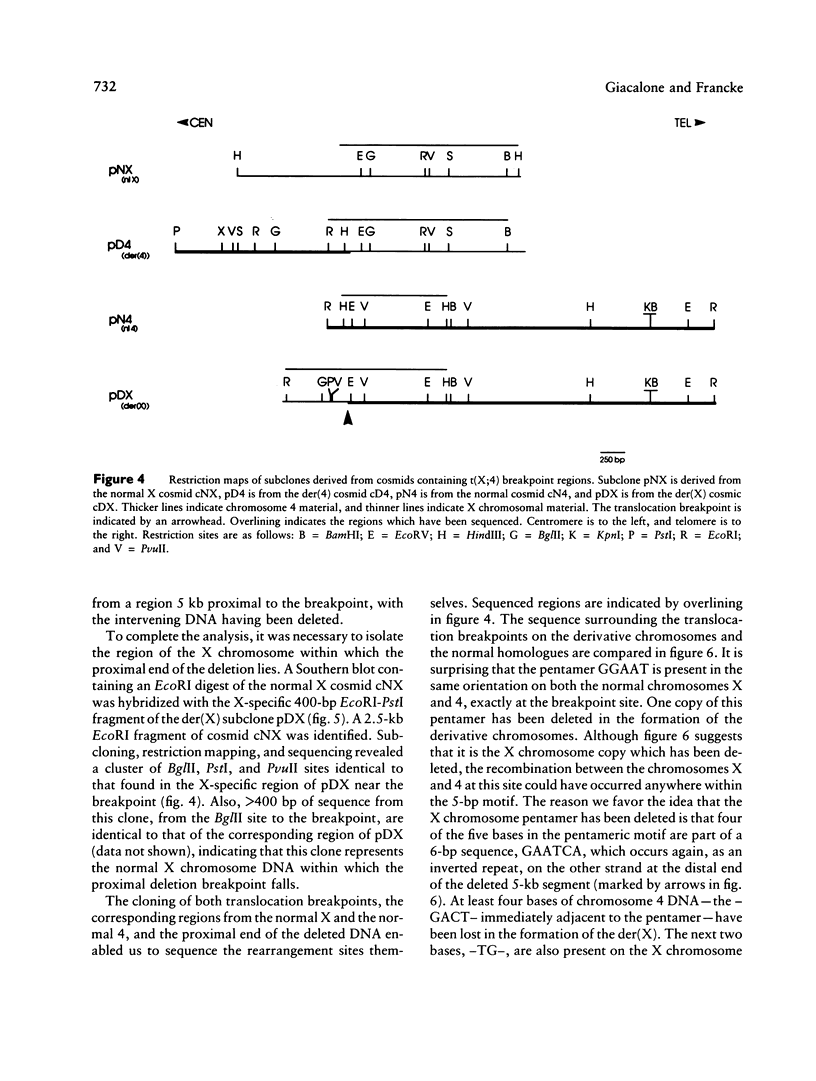
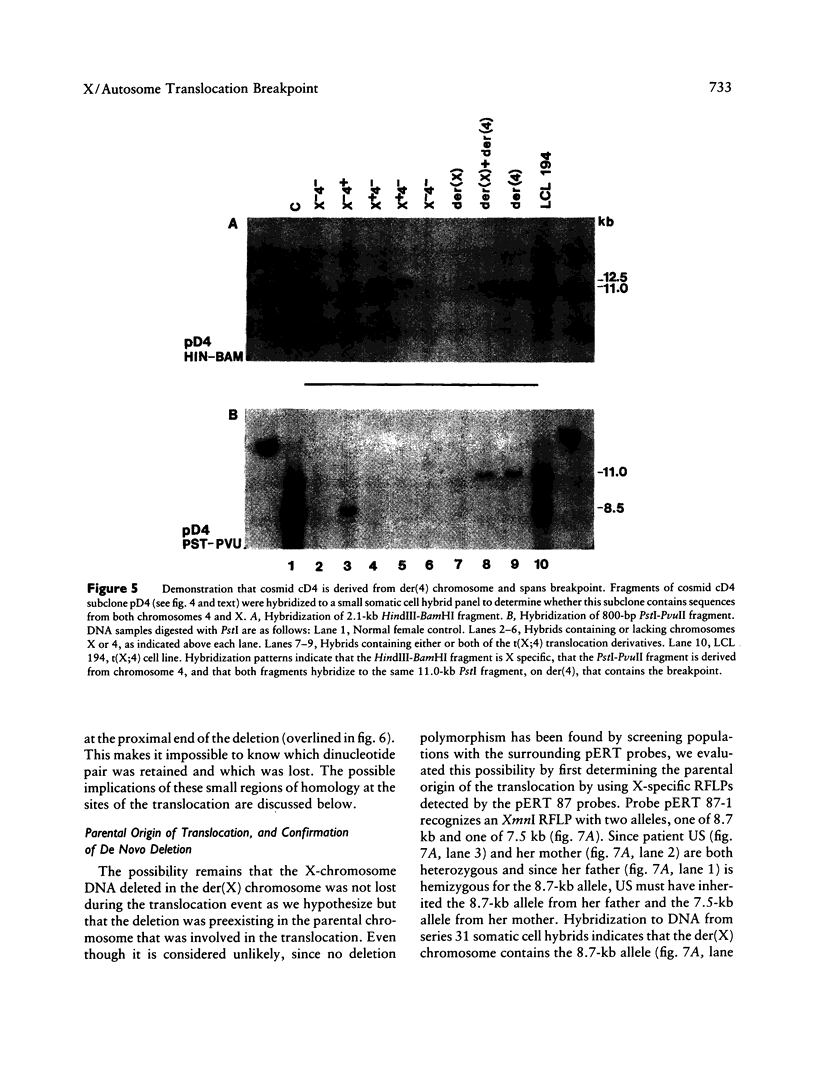
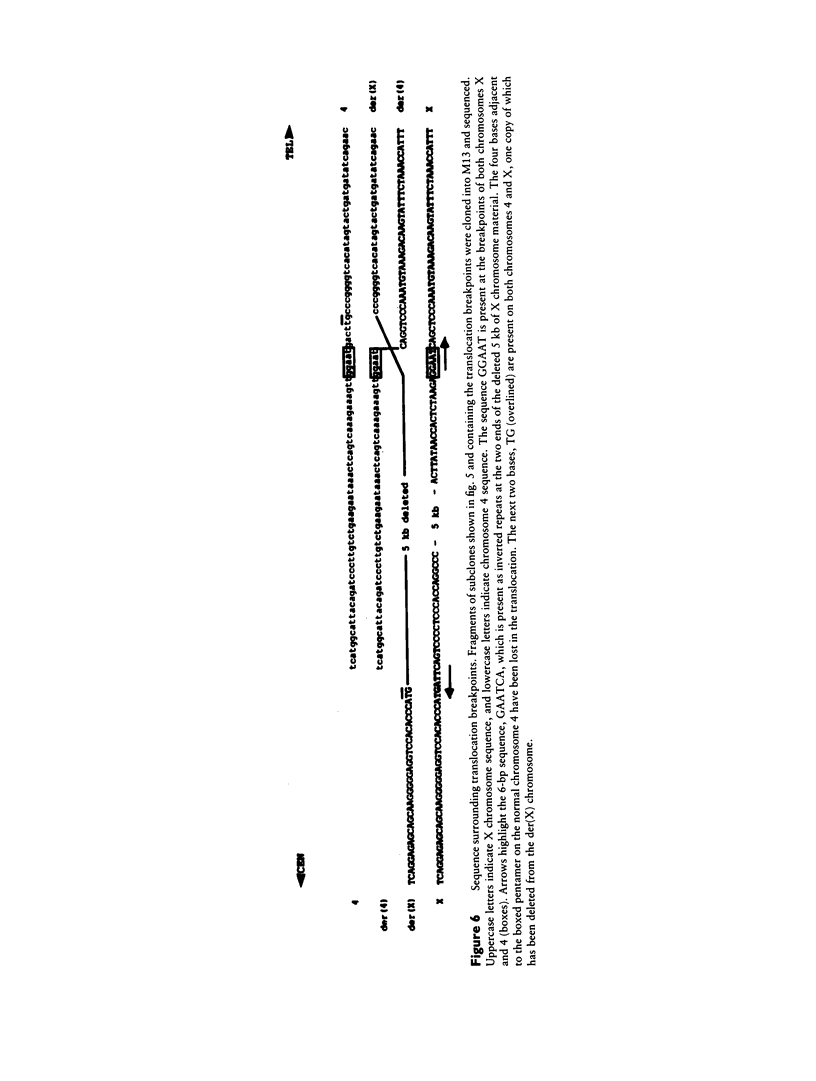
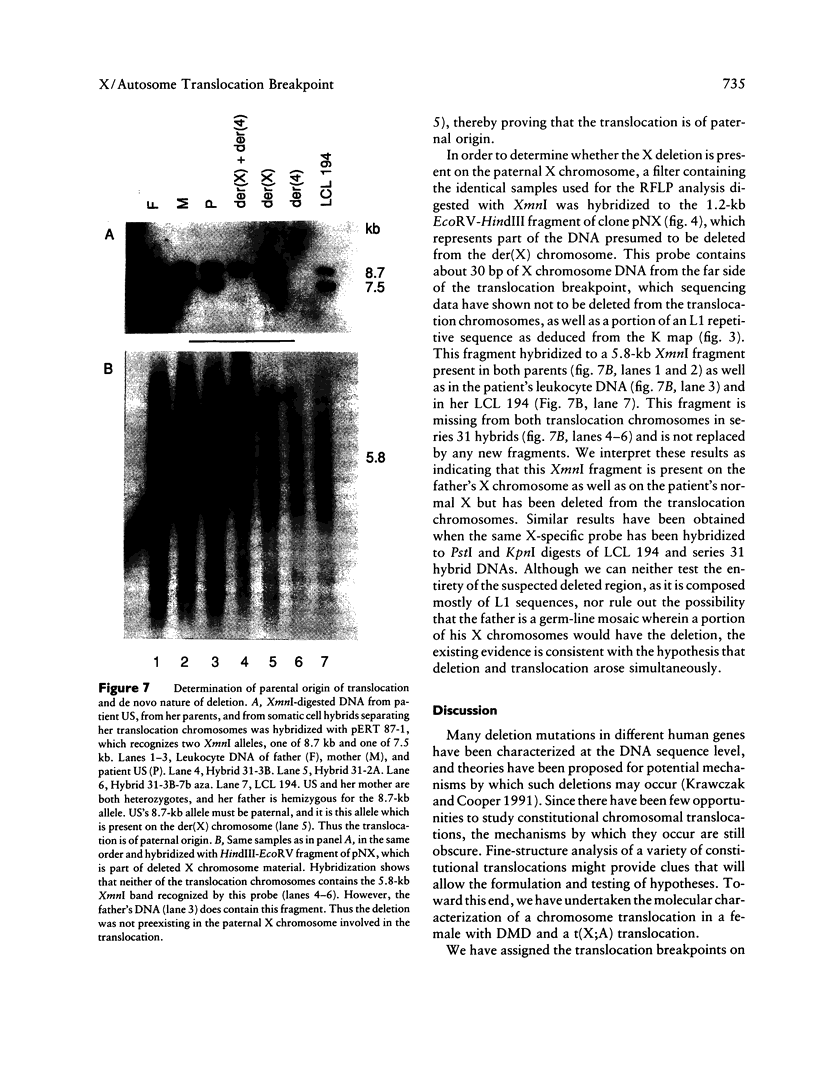
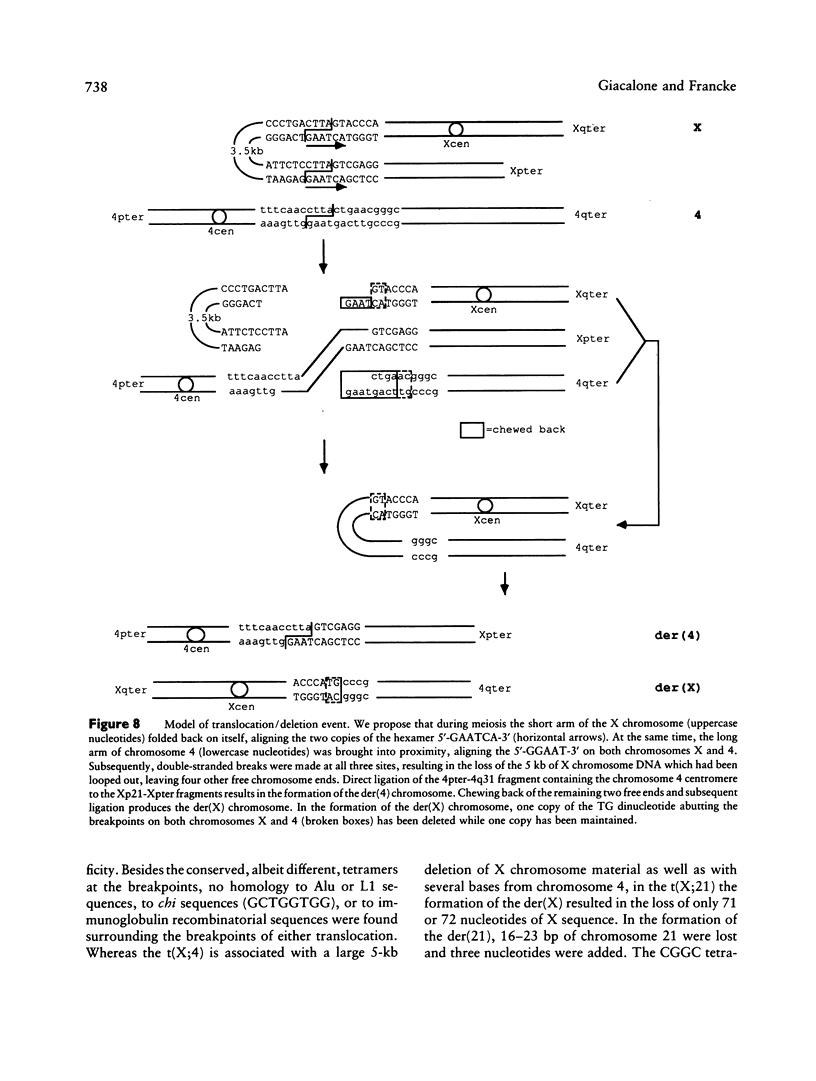
Reciprocal chromosome translocations are common de novo rearrangements that occur randomly throughout the human genome. To learn about causative mechanisms, we have cloned and sequenced the breakpoints of a cytologically balanced constitutional reciprocal translocation, t(X;4)(p21.2;q31.22), present in a girl with Duchenne muscular dystrophy (DMD). Physical mapping of the derivative chromosomes, after their separation in somatic cell hybrids, reveals that the translocation disrupts the DMD gene in Xp21 within the 18-kb intron 16. Restriction mapping and sequencing of clones that span both translocation breakpoints as well as the corresponding normal regions indicate the loss of approximately 5 kb in the formation of the derivative X chromosome, with 4-6 bp deleted from chromosome 4. RFLP and Southern analyses indicate that the de novo translocation is a paternal origin and that the father's X chromosome contains the DNA that is deleted in the derivative X. Most likely, deletion and translation arose simultaneously from a complex rearrangement event that involves three chromosomal breakpoints. Short regions of sequence homology were present at the three sites. A 5-bp sequence, GGAAT, found exactly at the translocation breakpoints on both normal chromosomes X and 4, has been preserved only on the der(4) chromosome. It is likely that the X-derived sequence GGAATCA has been lost in the formation of the der(X) chromosome, as it matches an inverted GAATCA sequence present on the opposite strand exactly at the other end of the deleted 5-kb fragment. These findings suggest a possible mechanism which may have juxtaposed the three sites and mediated sequence-specific breakage and recombination between nonhomologous chromosomes in male meiosis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., Bikker H., van Ommen G. J., de Vijlder J. J. Unusual scarcity of restriction site polymorphism in the human thyroglobulin gene. A linkage study suggesting autosomal dominance of a defective thyroglobulin allele. Hum Genet. 1984;67(3):301–305. doi: 10.1007/BF00291357. [DOI] [PubMed] [Google Scholar]
- Bjerglund Nielsen L., Nielsen I. M. Turner's syndrome and Duchenne muscular dystrophy in a girl with an X; autosome translocation. Ann Genet. 1984;27(3):173–177. [PubMed] [Google Scholar]
- Blonden L. A., Grootscholten P. M., den Dunnen J. T., Bakker E., Abbs S., Bobrow M., Boehm C., van Broeckhoven C., Baumbach L., Chamberlain J. 242 breakpoints in the 200-kb deletion-prone P20 region of the DMD gene are widely spread. Genomics. 1991 Jul;10(3):631–639. doi: 10.1016/0888-7543(91)90445-k. [DOI] [PubMed] [Google Scholar]
- Bodrug S. E., Ray P. N., Gonzalez I. L., Schmickel R. D., Sylvester J. E., Worton R. G. Molecular analysis of a constitutional X-autosome translocation in a female with muscular dystrophy. Science. 1987 Sep 25;237(4822):1620–1624. doi: 10.1126/science.3629260. [DOI] [PubMed] [Google Scholar]
- Bodrug S. E., Roberson J. R., Weiss L., Ray P. N., Worton R. G., Van Dyke D. L. Prenatal identification of a girl with a t(X;4)(p21;q35) translocation: molecular characterisation, paternal origin, and association with muscular dystrophy. J Med Genet. 1990 Jul;27(7):426–432. doi: 10.1136/jmg.27.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Mengle-Gaw L., Kees U. R., Spurr N., Lavenir I., Forster A., Rabbitts T. H. Alternating purine-pyrimidine tracts may promote chromosomal translocations seen in a variety of human lymphoid tumours. EMBO J. 1989 Sep;8(9):2621–2631. doi: 10.1002/j.1460-2075.1989.tb08402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boué A., Boué J., Gropp A. Cytogenetics of pregnancy wastage. Adv Hum Genet. 1985;14:1–57. doi: 10.1007/978-1-4615-9400-0_1. [DOI] [PubMed] [Google Scholar]
- Boyd Y., Buckle V. J. Cytogenetic heterogeneity of translocations associated with Duchenne muscular dystrophy. Clin Genet. 1986 Feb;29(2):108–115. doi: 10.1111/j.1399-0004.1986.tb01232.x. [DOI] [PubMed] [Google Scholar]
- Boyd Y., Buckle V., Holt S., Munro E., Hunter D., Craig I. Muscular dystrophy in girls with X;autosome translocations. J Med Genet. 1986 Dec;23(6):484–490. doi: 10.1136/jmg.23.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd Y., Cockburn D., Holt S., Munro E., Van Ommen G. J., Gillard B., Affara N., Ferguson-Smith M., Craig I. Mapping of 12 translocation breakpoints in the Xp21 region with respect to the locus for Duchenne muscular dystrophy. Cytogenet Cell Genet. 1988;48(1):28–34. doi: 10.1159/000132581. [DOI] [PubMed] [Google Scholar]
- Chamberlin J., Magenis R. E. Parental origin of de novo chromosome rearrangements. Hum Genet. 1980;53(3):343–347. doi: 10.1007/BF00287054. [DOI] [PubMed] [Google Scholar]
- Chandley A. C. The origin of chromosomal aberrations in man and their potential for survival and reproduction in the adult human population. Ann Genet. 1981;24(1):5–11. [PubMed] [Google Scholar]
- Darras B. T., Blattner P., Harper J. F., Spiro A. J., Alter S., Francke U. Intragenic deletions in 21 Duchenne muscular dystrophy (DMD)/Becker muscular dystrophy (BMD) families studied with the dystrophin cDNA: location of breakpoints on HindIII and BglII exon-containing fragment maps, meiotic and mitotic origin of the mutations. Am J Hum Genet. 1988 Nov;43(5):620–629. [PMC free article] [PubMed] [Google Scholar]
- Den Dunnen J. T., Grootscholten P. M., Bakker E., Blonden L. A., Ginjaar H. B., Wapenaar M. C., van Paassen H. M., van Broeckhoven C., Pearson P. L., van Ommen G. J. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet. 1989 Dec;45(6):835–847. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Francke U., Darras B. T., Hersh J. H., Berg B. O., Miller R. G. Brother/sister pairs affected with early-onset, progressive muscular dystrophy: molecular studies reveal etiologic heterogeneity. Am J Hum Genet. 1989 Jul;45(1):63–72. [PMC free article] [PubMed] [Google Scholar]
- Francke U. High-resolution ideograms of trypsin-Giemsa banded human chromosomes. Cytogenet Cell Genet. 1981;31(1):24–32. doi: 10.1159/000131622. [DOI] [PubMed] [Google Scholar]
- Francke U., Ochs H. D., de Martinville B., Giacalone J., Lindgren V., Distèche C., Pagon R. A., Hofker M. H., van Ommen G. J., Pearson P. L. Minor Xp21 chromosome deletion in a male associated with expression of Duchenne muscular dystrophy, chronic granulomatous disease, retinitis pigmentosa, and McLeod syndrome. Am J Hum Genet. 1985 Mar;37(2):250–267. [PMC free article] [PubMed] [Google Scholar]
- Francke U., Oliver N. Quantitative analysis of high-resolution trypsin-giemsa bands on human prometaphase chromosomes. Hum Genet. 1978 Dec 18;45(2):137–165. doi: 10.1007/BF00286957. [DOI] [PubMed] [Google Scholar]
- Funderburk S. J., Spence M. A., Sparkes R. S. Mental retardation associated with "balanced" chromosome rearrangements. Am J Hum Genet. 1977 Mar;29(2):136–141. [PMC free article] [PubMed] [Google Scholar]
- Gerstein R. M., Frankel W. N., Hsieh C. L., Durdik J. M., Rath S., Coffin J. M., Nisonoff A., Selsing E. Isotype switching of an immunoglobulin heavy chain transgene occurs by DNA recombination between different chromosomes. Cell. 1990 Nov 2;63(3):537–548. doi: 10.1016/0092-8674(90)90450-s. [DOI] [PubMed] [Google Scholar]
- Jacobs P. A. Correlation between euploid structural chromosome rearrangements and mental subnormality in humans. Nature. 1974 May 10;249(453):164–165. doi: 10.1038/249164a0. [DOI] [PubMed] [Google Scholar]
- Kean V. M., Macleod H. L., Thompson M. W., Ray P. N., Verellen-Dumoulin C., Worton R. G. Paternal inheritance of translocation chromosomes in a t(X;21) patient with X linked muscular dystrophy. J Med Genet. 1986 Dec;23(6):491–493. doi: 10.1136/jmg.23.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Kunkel L. M. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem. 1990 Mar 15;265(8):4560–4566. [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Cooper D. N. Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet. 1991 Mar;86(5):425–441. doi: 10.1007/BF00194629. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Monaco A. P., Middlesworth W., Ochs H. D., Latt S. A. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4778–4782. doi: 10.1073/pnas.82.14.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. R., England S. B., Speer A., Marsden R. F., Bloomfield J. F., Roche A. L., Cross G. S., Mountford R. C., Smith T. J., Davies K. E. Sequences of junction fragments in the deletion-prone region of the dystrophin gene. Genomics. 1991 May;10(1):57–67. doi: 10.1016/0888-7543(91)90484-v. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Genetic evidence for the inactivation of a human autosomal locus attached to an inactive X chromosome. Am J Hum Genet. 1982 Sep;34(5):811–817. [PMC free article] [PubMed] [Google Scholar]
- Moser H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet. 1984;66(1):17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991 May;87(1):81–83. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- Pai G. S., Thomas G. H., Mahoney W., Migeon B. R. Complex chromosome rearrangements. Report of a new case and literature review. Clin Genet. 1980 Dec;18(6):436–444. doi: 10.1111/j.1399-0004.1980.tb01790.x. [DOI] [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quack B., Speed R. M., Luciani J. M., Noel B., Guichaoua M., Chandley A. C. Meiotic analysis of two human reciprocal X-autosome translocations. Cytogenet Cell Genet. 1988;48(1):43–47. doi: 10.1159/000132583. [DOI] [PubMed] [Google Scholar]
- Ribeiro M. C., Melaragno M. I., Schmidt B., Brunoni D., Gabbai A. A., Hackel C. Duchenne muscular dystrophy in a girl with an (X;15) translocation. Am J Med Genet. 1986 Oct;25(2):231–236. doi: 10.1002/ajmg.1320250205. [DOI] [PubMed] [Google Scholar]
- Richards C. S., Watkins S. C., Hoffman E. P., Schneider N. R., Milsark I. W., Katz K. S., Cook J. D., Kunkel L. M., Cortada J. M. Skewed X inactivation in a female MZ twin results in Duchenne muscular dystrophy. Am J Hum Genet. 1990 Apr;46(4):672–681. [PMC free article] [PubMed] [Google Scholar]
- Robinson D. O., Boyd Y., Cockburn D., Collinson M. N., Craig I., Jacobs P. A. The parental origin of de novo X-autosome translocations in females with Duchenne muscular dystrophy revealed by M27 beta methylation analysis. Genet Res. 1990 Oct-Dec;56(2-3):135–140. doi: 10.1017/s0016672300035217. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wapenaar M. C., Kievits T., Hart K. A., Abbs S., Blonden L. A., den Dunnen J. T., Grootscholten P. M., Bakker E., Verellen-Dumoulin C., Bobrow M. A deletion hot spot in the Duchenne muscular dystrophy gene. Genomics. 1988 Feb;2(2):101–108. doi: 10.1016/0888-7543(88)90090-0. [DOI] [PubMed] [Google Scholar]
- Warburton D. Outcome of cases of de novo structural rearrangements diagnosed at amniocentesis. Prenat Diagn. 1984 Spring;4(Spec No):69–80. doi: 10.1002/pd.1970040706. [DOI] [PubMed] [Google Scholar]
- de Martinville B., Kunkel L. M., Bruns G., Morlé F., Koenig M., Mandel J. L., Horwich A., Latt S. A., Gusella J. F., Housman D. Localization of DNA sequences in region Xp21 of the human X chromosome: search for molecular markers close to the Duchenne muscular dystrophy locus. Am J Hum Genet. 1985 Mar;37(2):235–249. [PMC free article] [PubMed] [Google Scholar]