Abstract
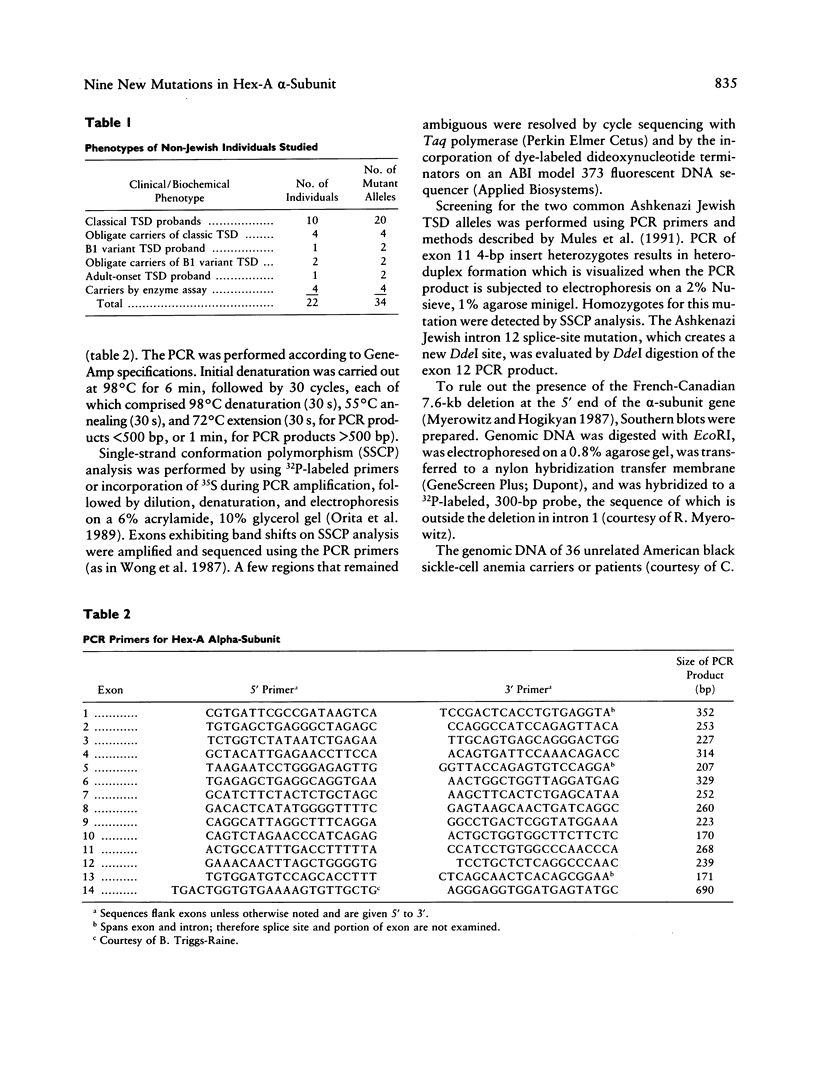
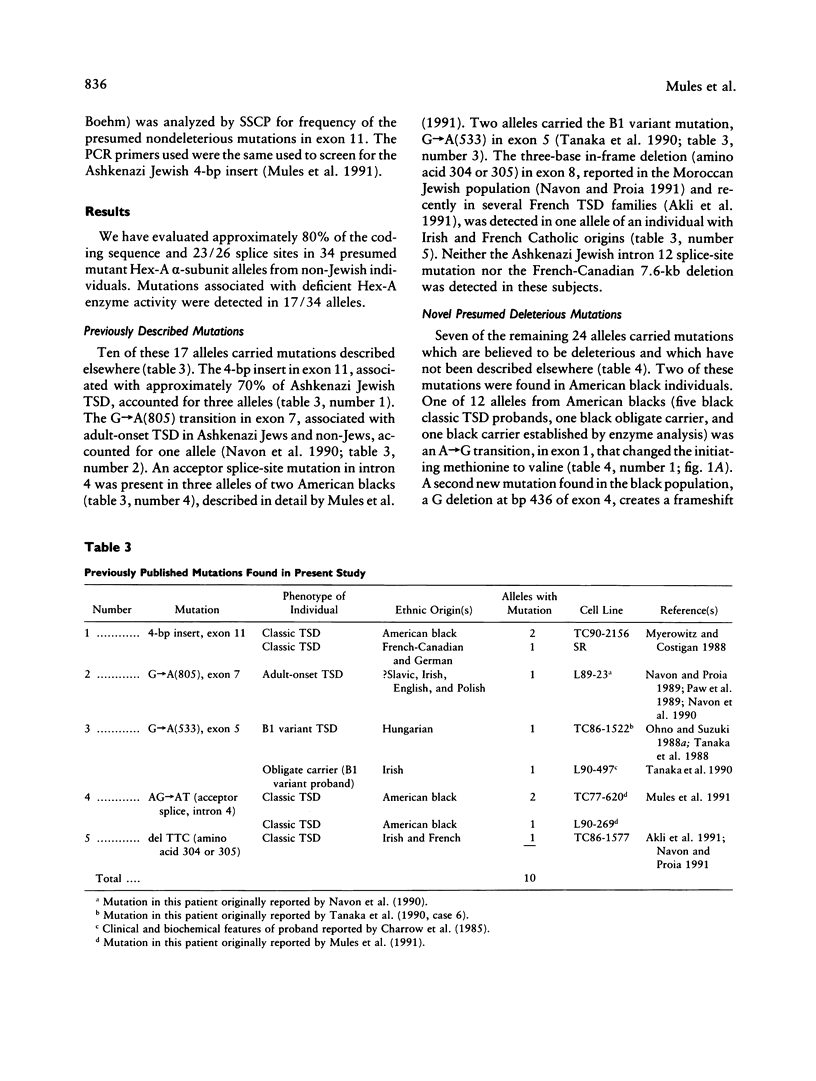
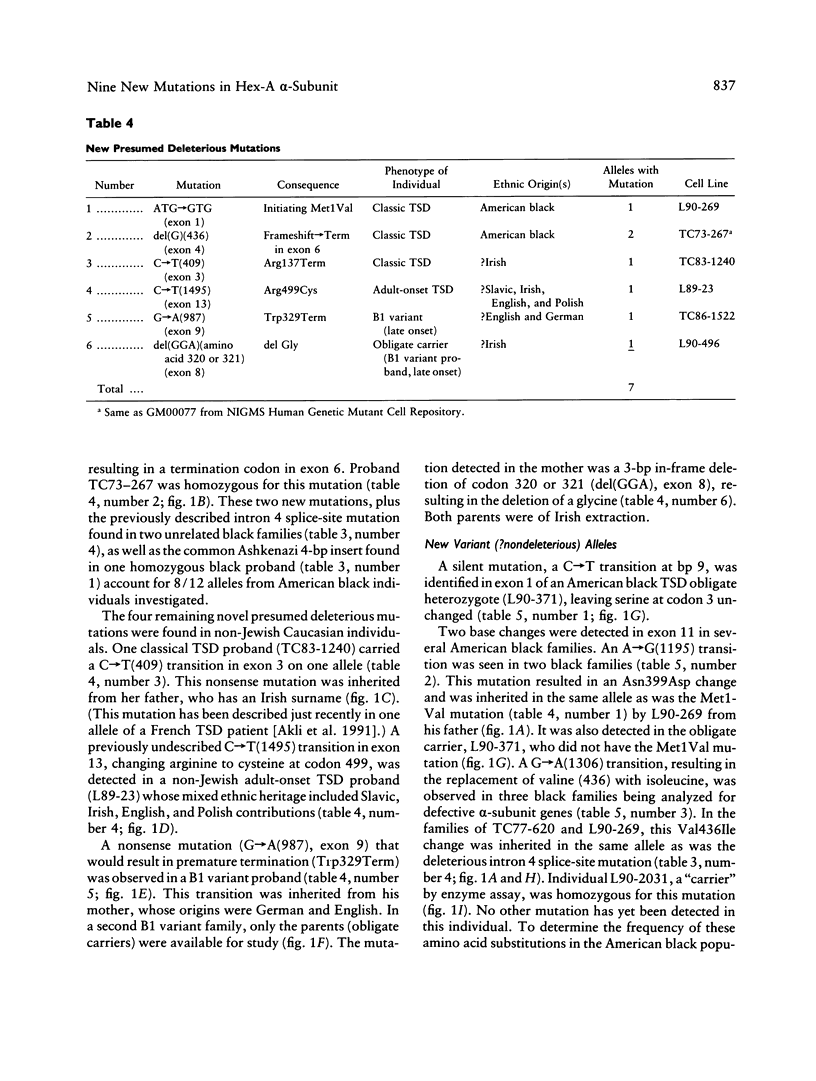
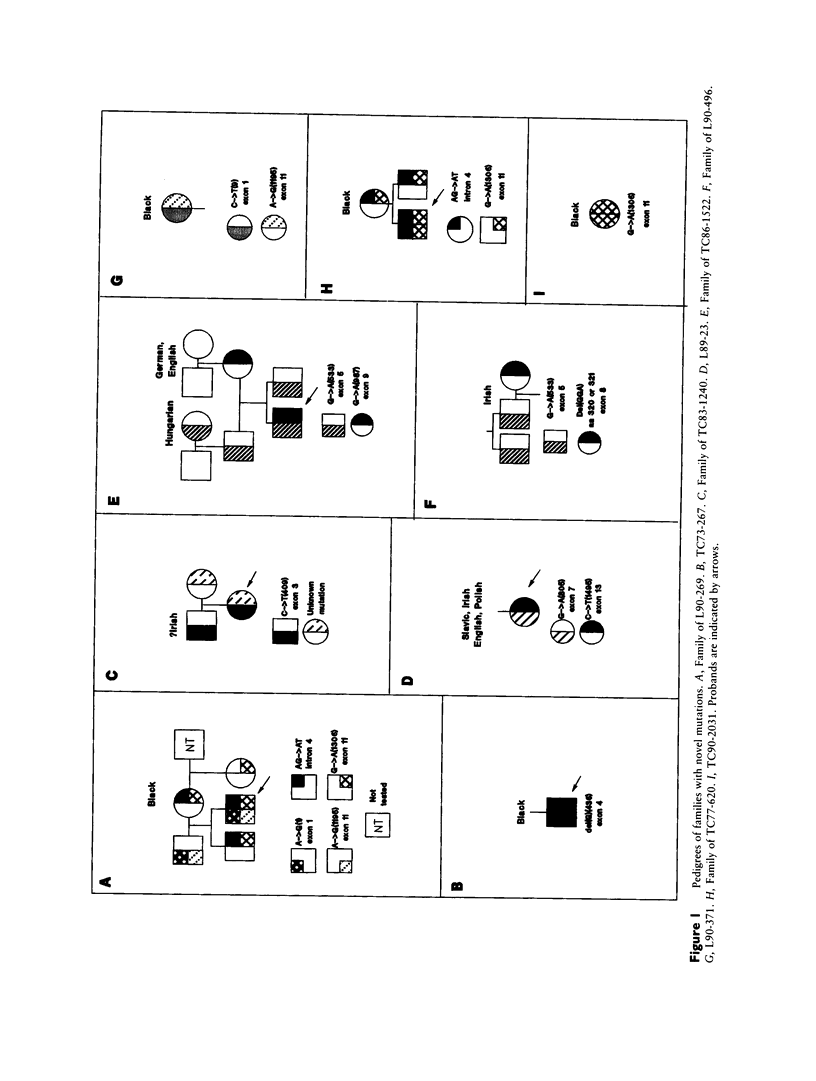
Initial investigations demonstrated that only 3/34 "Tay-Sachs chromosomes" in 22 unrelated, non-Jewish patients or carriers of some form of GM2-gangliosidosis (7 black and 15 non-Jewish Caucasian) had either of the two mutations commonly found in the Jewish population. To determine the nature and incidence of the alterations in this non-Jewish population we have utilized PCR, single-strand conformation polymorphism analysis and sequencing to detect new mutations in genomic DNA. Fourteen primer sets have been utilized to analyze 80% of the coding region and 23/26 splice sites of the gene coding for the alpha chain of hexosaminidase A. Presumed deleterious mutations were discovered in 17/34 chromosomes believed to be carrying a beta-hexosaminidase A alpha-subunit gene mutation. Ten had abnormalities which have been described previously. In the remaining 24 Tay-Sachs disease alleles, six novel mutations predicted to be deleterious were discovered. These include two small deletions (a single-base frameshift and a three-base deletion removing an amino acid), two different nonsense mutations, an initiation codon mutation (ATG----GTG), and a missense mutation (Arg499Cys) in a highly conserved residue. In addition, three presumed nondeleterious mutations were found.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akli S., Chelly J., Lacorte J. M., Poenaru L., Kahn A. Seven novel Tay-Sachs mutations detected by chemical mismatch cleavage of PCR-amplified cDNA fragments. Genomics. 1991 Sep;11(1):124–134. doi: 10.1016/0888-7543(91)90109-r. [DOI] [PubMed] [Google Scholar]
- Arpaia E., Dumbrille-Ross A., Maler T., Neote K., Tropak M., Troxel C., Stirling J. L., Pitts J. S., Bapat B., Lamhonwah A. M. Identification of an altered splice site in Ashkenazi Tay-Sachs disease. Nature. 1988 May 5;333(6168):85–86. doi: 10.1038/333085a0. [DOI] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Charrow J., Inui K., Wenger D. A. Late onset GM2 gangliosidosis: an alpha-locus genetic compound with near normal hexosaminidase activity. Clin Genet. 1985 Jan;27(1):78–84. doi: 10.1111/j.1399-0004.1985.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Graham T. R., Zassenhaus H. P., Kaplan A. Molecular cloning of the cDNA which encodes beta-N-acetylhexosaminidase A from Dictyostelium discoideum. Complete amino acid sequence and homology with the human enzyme. J Biol Chem. 1988 Nov 15;263(32):16823–16829. [PubMed] [Google Scholar]
- Grebner E. E., Tomczak J. Distribution of three alpha-chain beta-hexosaminidase A mutations among Tay-Sachs carriers. Am J Hum Genet. 1991 Mar;48(3):604–607. [PMC free article] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Buerstedde J. M., Sommer S. S. Functionally important regions of the factor IX gene have a low rate of polymorphism and a high rate of mutation in the dinucleotide CpG. Am J Hum Genet. 1989 Sep;45(3):448–457. [PMC free article] [PubMed] [Google Scholar]
- Lau M. M., Neufeld E. F. A frameshift mutation in a patient with Tay-Sachs disease causes premature termination and defective intracellular transport of the alpha-subunit of beta-hexosaminidase. J Biol Chem. 1989 Dec 15;264(35):21376–21380. [PubMed] [Google Scholar]
- Mules E. H., Dowling C. E., Petersen M. B., Kazazian H. H., Jr, Thomas G. H. A novel mutation in the invariant AG of the acceptor splice site of intron 4 of the beta-hexosaminidase alpha-subunit gene in two unrelated American black GM2-gangliosidosis (Tay-Sachs disease) patients. Am J Hum Genet. 1991 Jun;48(6):1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R., Costigan F. C. The major defect in Ashkenazi Jews with Tay-Sachs disease is an insertion in the gene for the alpha-chain of beta-hexosaminidase. J Biol Chem. 1988 Dec 15;263(35):18587–18589. [PubMed] [Google Scholar]
- Myerowitz R., Hogikyan N. D. A deletion involving Alu sequences in the beta-hexosaminidase alpha-chain gene of French Canadians with Tay-Sachs disease. J Biol Chem. 1987 Nov 15;262(32):15396–15399. [PubMed] [Google Scholar]
- Myerowitz R. Splice junction mutation in some Ashkenazi Jews with Tay-Sachs disease: evidence against a single defect within this ethnic group. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3955–3959. doi: 10.1073/pnas.85.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Muscillo M., Ohno K., Hoffman A. J., Suzuki K. A point mutation in the coding sequence of the beta-hexosaminidase alpha gene results in defective processing of the enzyme protein in an unusual GM2-gangliosidosis variant. J Neurochem. 1988 Sep;51(3):984–987. doi: 10.1111/j.1471-4159.1988.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Nakano T., Nanba E., Tanaka A., Ohno K., Suzuki Y., Suzuki K. A new point mutation within exon 5 of beta-hexosaminidase alpha gene in a Japanese infant with Tay-Sachs disease. Ann Neurol. 1990 May;27(5):465–473. doi: 10.1002/ana.410270503. [DOI] [PubMed] [Google Scholar]
- Navon R., Kolodny E. H., Mitsumoto H., Thomas G. H., Proia R. L. Ashkenazi-Jewish and non-Jewish adult GM2 gangliosidosis patients share a common genetic defect. Am J Hum Genet. 1990 Apr;46(4):817–821. [PMC free article] [PubMed] [Google Scholar]
- Navon R., Proia R. L. The mutations in Ashkenazi Jews with adult GM2 gangliosidosis, the adult form of Tay-Sachs disease. Science. 1989 Mar 17;243(4897):1471–1474. doi: 10.1126/science.2522679. [DOI] [PubMed] [Google Scholar]
- Ohno K., Suzuki K. Mutation in GM2-gangliosidosis B1 variant. J Neurochem. 1988 Jan;50(1):316–318. doi: 10.1111/j.1471-4159.1988.tb13266.x. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Paw B. H., Kaback M. M., Neufeld E. F. Molecular basis of adult-onset and chronic GM2 gangliosidoses in patients of Ashkenazi Jewish origin: substitution of serine for glycine at position 269 of the alpha-subunit of beta-hexosaminidase. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2413–2417. doi: 10.1073/pnas.86.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paw B. H., Moskowitz S. M., Uhrhammer N., Wright N., Kaback M. M., Neufeld E. F. Juvenile GM2 gangliosidosis caused by substitution of histidine for arginine at position 499 or 504 of the alpha-subunit of beta-hexosaminidase. J Biol Chem. 1990 Jun 5;265(16):9452–9457. [PubMed] [Google Scholar]
- Paw B. H., Tieu P. T., Kaback M. M., Lim J., Neufeld E. F. Frequency of three Hex A mutant alleles among Jewish and non-Jewish carriers identified in a Tay-Sachs screening program. Am J Hum Genet. 1990 Oct;47(4):698–705. [PMC free article] [PubMed] [Google Scholar]
- Paw B. H., Wood L. C., Neufeld E. F. A third mutation at the CpG dinucleotide of codon 504 and a silent mutation at codon 506 of the HEX A gene. Am J Hum Genet. 1991 Jun;48(6):1139–1146. [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Ohno K., Sandhoff K., Maire I., Kolodny E. H., Brown A., Suzuki K. GM2-gangliosidosis B1 variant: analysis of beta-hexosaminidase alpha gene abnormalities in seven patients. Am J Hum Genet. 1990 Feb;46(2):329–339. [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Ohno K., Suzuki K. GM2-gangliosidosis B1 variant: a wide geographic and ethnic distribution of the specific beta-hexosaminidase alpha chain mutation originally identified in a Puerto Rican patient. Biochem Biophys Res Commun. 1988 Oct 31;156(2):1015–1019. doi: 10.1016/s0006-291x(88)80945-8. [DOI] [PubMed] [Google Scholar]
- Triggs-Raine B. L., Feigenbaum A. S., Natowicz M., Skomorowski M. A., Schuster S. M., Clarke J. T., Mahuran D. J., Kolodny E. H., Gravel R. A. Screening for carriers of Tay-Sachs disease among Ashkenazi Jews. A comparison of DNA-based and enzyme-based tests. N Engl J Med. 1990 Jul 5;323(1):6–12. doi: 10.1056/NEJM199007053230102. [DOI] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]


