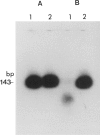
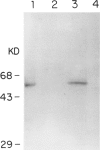
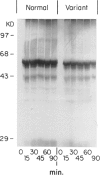
Abstract
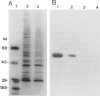
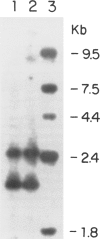
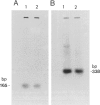
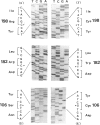
Among a large number of glucose-6-phosphate dehydrogenase (G6PD) variants associated with different severity of clinical manifestations, enzyme deficiency, and kinetic abnormalities found in humans, only one variant exhibits no measurable activity and lacks an immunologically cross-reacting material in blood cells and other tissues. The mRNA content of the patient's lymphoblastoid cells was found to be normal, and the size of mRNA was also normal (i.e., approximately 2.4 kb). Western blot hybridization indicated that the patient's cells did not produce cross-reacting material. The variant mRNA was reverse transcribed and amplified by PCR. Nucleotide sequencing of the variant cDNA showed the existence of three nucleotide base changes, i.e., a C----G at nucleotide 317 (counting from adenine of the initiation codon), which should cause Ser----Cys substitution at the 106th position (counting from the initiation Met); a C----T at nucleotide 544, which induces the Arg----Trp at the 182d position; and a C----T at nucleotide 592, which induces Arg----Cys at the 198th position of the protein. The existence of three mutation sites was confirmed by sequencing of selected regions of the variant gene. No base deletion or frameshift mutation was found in the variant cDNA. No nucleotide change was detected in the extended 5' region, which included the most distal cap site. When the variant cDNA was expressed in Escherichia coli, the G6PD activity was approximately 2% of that expressed by the normal cDNA, and cross-reacting material was undetectable. However, when the variant mRNA was expressed in the in vitro translation system of rabbit reticulocytes, the variant protein was produced. These results suggest that extremely rapid in vivo degradation or precipitation of the variant enzyme induced by the three amino acid substitutions could be the major cause of the molecular deficiency.
Full text
PDF



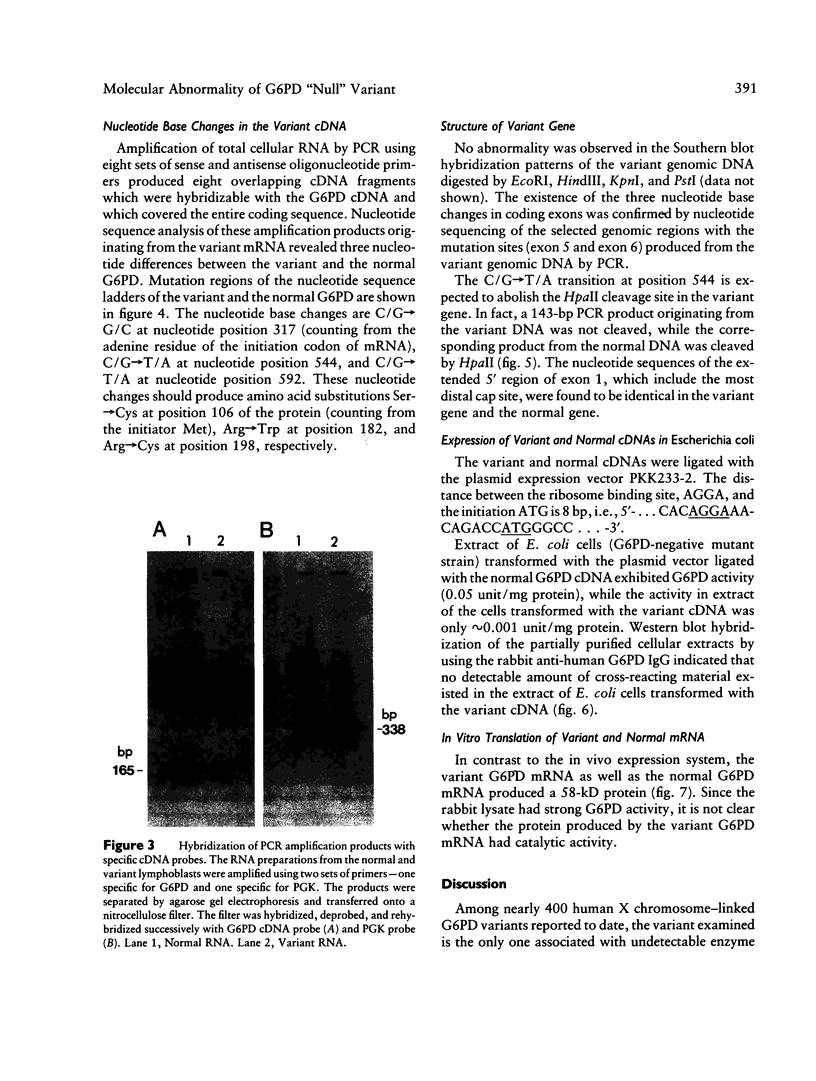
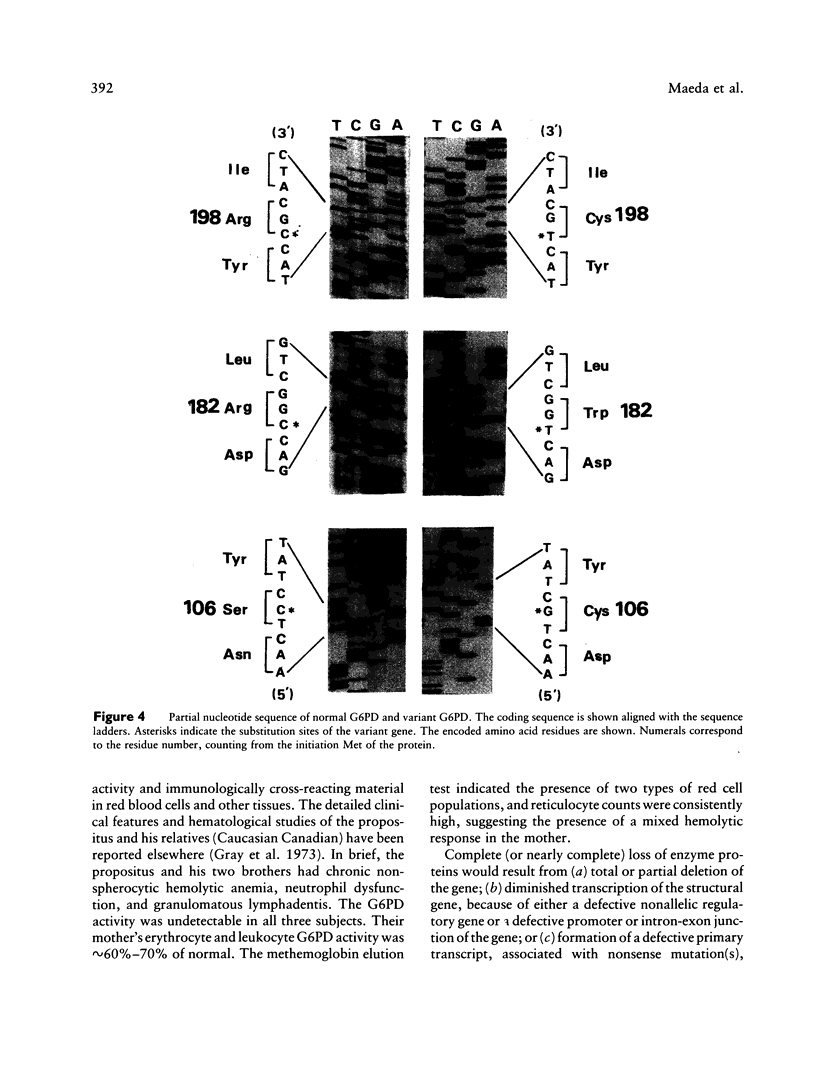
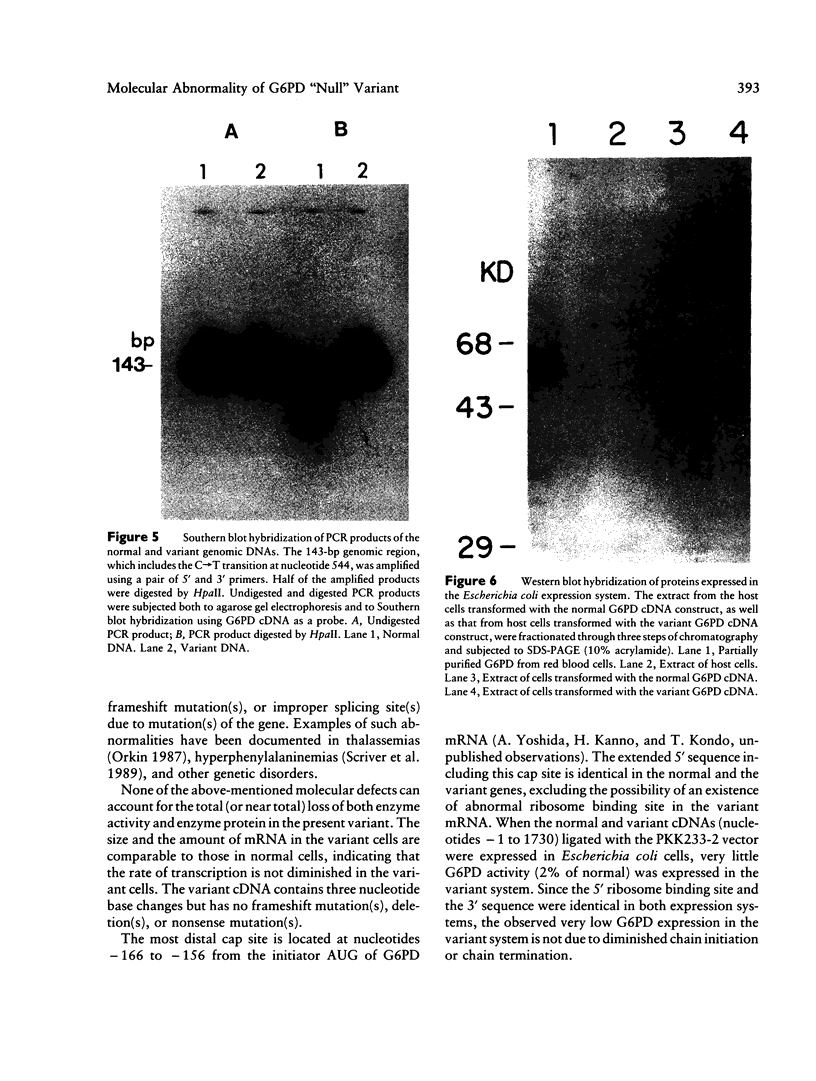
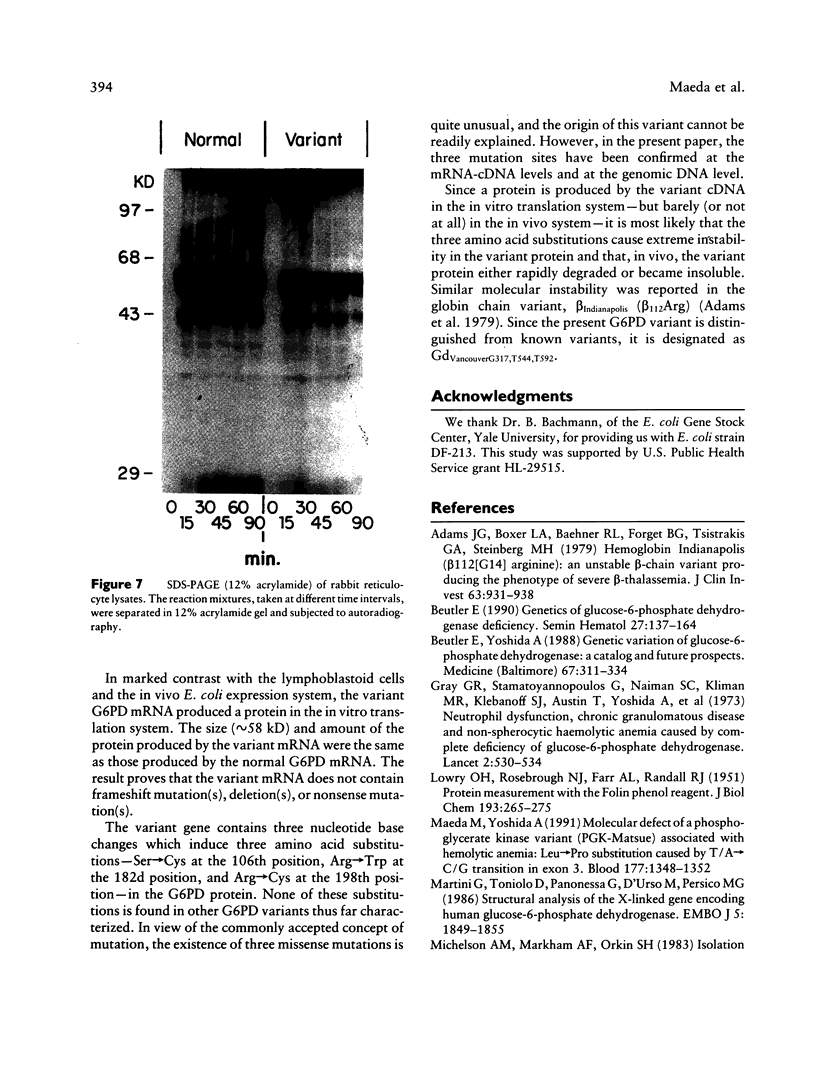

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. G., 3rd, Boxer L. A., Baehner R. L., Forget B. G., Tsistrakis G. A., Steinberg M. H. Hemoglobin Indianapolis (beta 112[G14] arginine). An unstable beta-chain variant producing the phenotype of severe beta-thalassemia. J Clin Invest. 1979 May;63(5):931–938. doi: 10.1172/JCI109393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. G., 3rd, Boxer L. A., Baehner R. L., Forget B. G., Tsistrakis G. A., Steinberg M. H. Hemoglobin Indianapolis (beta 112[G14] arginine). An unstable beta-chain variant producing the phenotype of severe beta-thalassemia. J Clin Invest. 1979 May;63(5):931–938. doi: 10.1172/JCI109393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. The genetics of glucose-6-phosphate dehydrogenase deficiency. Semin Hematol. 1990 Apr;27(2):137–164. [PubMed] [Google Scholar]
- Beutler E. The genetics of glucose-6-phosphate dehydrogenase deficiency. Semin Hematol. 1990 Apr;27(2):137–164. [PubMed] [Google Scholar]
- Beutler E., Yoshida A. Genetic variation of glucose-6-phosphate dehydrogenase: a catalog and future prospects. Medicine (Baltimore) 1988 Sep;67(5):311–334. doi: 10.1097/00005792-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Beutler E., Yoshida A. Genetic variation of glucose-6-phosphate dehydrogenase: a catalog and future prospects. Medicine (Baltimore) 1988 Sep;67(5):311–334. doi: 10.1097/00005792-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Gray G. R., Stamatoyannopoulos G., Naiman S. C., Kliman M. R., Klebanoff S. J., Austin T., Yoshida A., Robinson G. C. Neutrophil dysfunction, chronic granulomatous disease, and non-spherocytic haemolytic anaemia caused by complete deficiency of glucose-6-phosphate dehydrogenase. Lancet. 1973 Sep 8;2(7828):530–534. doi: 10.1016/s0140-6736(73)92350-7. [DOI] [PubMed] [Google Scholar]
- Gray G. R., Stamatoyannopoulos G., Naiman S. C., Kliman M. R., Klebanoff S. J., Austin T., Yoshida A., Robinson G. C. Neutrophil dysfunction, chronic granulomatous disease, and non-spherocytic haemolytic anaemia caused by complete deficiency of glucose-6-phosphate dehydrogenase. Lancet. 1973 Sep 8;2(7828):530–534. doi: 10.1016/s0140-6736(73)92350-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maeda M., Yoshida A. Molecular defect of a phosphoglycerate kinase variant (PGK-Matsue) associated with hemolytic anemia: Leu----Pro substitution caused by T/A----C/G transition in exon 3. Blood. 1991 Mar 15;77(6):1348–1352. [PubMed] [Google Scholar]
- Maeda M., Yoshida A. Molecular defect of a phosphoglycerate kinase variant (PGK-Matsue) associated with hemolytic anemia: Leu----Pro substitution caused by T/A----C/G transition in exon 3. Blood. 1991 Mar 15;77(6):1348–1352. [PubMed] [Google Scholar]
- Martini G., Toniolo D., Vulliamy T., Luzzatto L., Dono R., Viglietto G., Paonessa G., D'Urso M., Persico M. G. Structural analysis of the X-linked gene encoding human glucose 6-phosphate dehydrogenase. EMBO J. 1986 Aug;5(8):1849–1855. doi: 10.1002/j.1460-2075.1986.tb04436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini G., Toniolo D., Vulliamy T., Luzzatto L., Dono R., Viglietto G., Paonessa G., D'Urso M., Persico M. G. Structural analysis of the X-linked gene encoding human glucose 6-phosphate dehydrogenase. EMBO J. 1986 Aug;5(8):1849–1855. doi: 10.1002/j.1460-2075.1986.tb04436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Markham A. F., Orkin S. H. Isolation and DNA sequence of a full-length cDNA clone for human X chromosome-encoded phosphoglycerate kinase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):472–476. doi: 10.1073/pnas.80.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Yoshida A. Glucose 6-phosphate dehydrogenase of human erythrocytes. I. Purification and characterization of normal (B+) enzyme. J Biol Chem. 1966 Nov 10;241(21):4966–4976. [PubMed] [Google Scholar]