Abstract
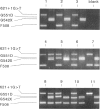
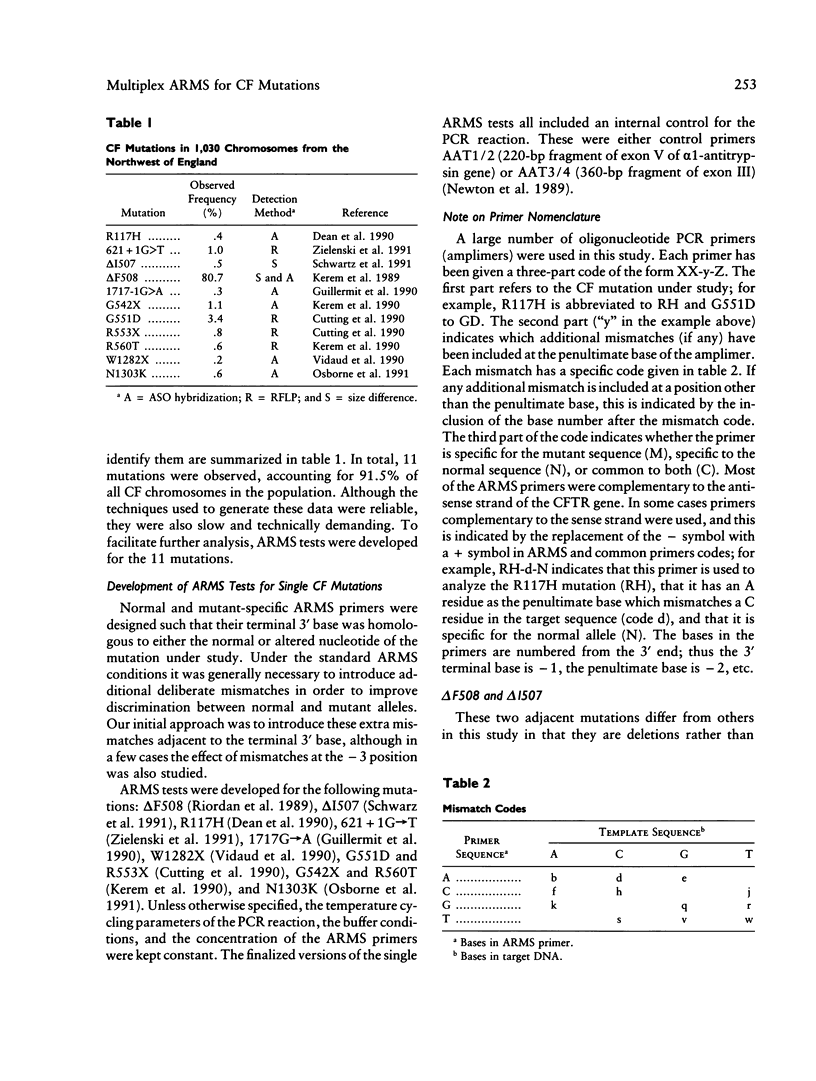
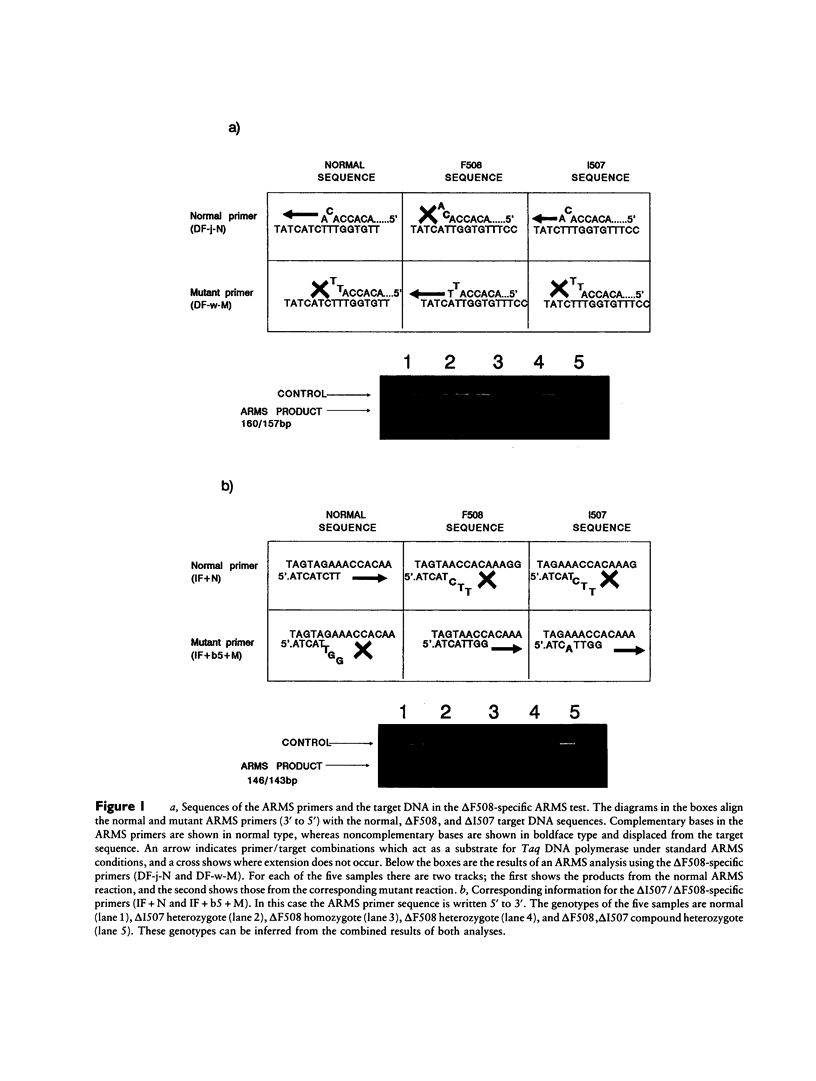
The amplification refractory mutation system (ARMS) is a simple, rapid and reliable method for the detection of any mutation involving single base changes or small deletions. We have applied ARMS methodology to the detection of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Single ARMS tests have been developed for 11 CFTR mutations found in the northwest of England. ARMS reactions for the most common mutations have been multiplexed to give a test which will detect the presence of the delta F508, G551D, G542X, and 621 + 1G----T mutations in a DNA sample. The multiplex test has been validated by the analysis of over 500 previously genotyped samples and has been found to be completely accurate. The rapid detection of the most common mutations has enabled early molecular confirmation of suspected cystic fibrosis in neonates, rapid typing of cystic fibrosis patients and their relatives, and testing of sperm and egg donors.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballabio A., Gibbs R. A., Caskey C. T. PCR test for cystic fibrosis deletion. Nature. 1990 Jan 18;343(6255):220–220. doi: 10.1038/343220a0. [DOI] [PubMed] [Google Scholar]
- Chehab F. F., Doherty M., Cai S. P., Kan Y. W., Cooper S., Rubin E. M. Detection of sickle cell anaemia and thalassaemias. Nature. 1987 Sep 24;329(6137):293–294. doi: 10.1038/329293b0. [DOI] [PubMed] [Google Scholar]
- Cutting G. R., Kasch L. M., Rosenstein B. J., Zielenski J., Tsui L. C., Antonarakis S. E., Kazazian H. H., Jr A cluster of cystic fibrosis mutations in the first nucleotide-binding fold of the cystic fibrosis conductance regulator protein. Nature. 1990 Jul 26;346(6282):366–369. doi: 10.1038/346366a0. [DOI] [PubMed] [Google Scholar]
- Dean M., White M. B., Amos J., Gerrard B., Stewart C., Khaw K. T., Leppert M. Multiple mutations in highly conserved residues are found in mildly affected cystic fibrosis patients. Cell. 1990 Jun 1;61(5):863–870. doi: 10.1016/0092-8674(90)90196-l. [DOI] [PubMed] [Google Scholar]
- Guillermit H., Fanen P., Ferec C. A 3' splice site consensus sequence mutation in the cystic fibrosis gene. Hum Genet. 1990 Sep;85(4):450–453. doi: 10.1007/BF02428306. [DOI] [PubMed] [Google Scholar]
- Haliassos A., Chomel J. C., Tesson L., Baudis M., Kruh J., Kaplan J. C., Kitzis A. Modification of enzymatically amplified DNA for the detection of point mutations. Nucleic Acids Res. 1989 May 11;17(9):3606–3606. doi: 10.1093/nar/17.9.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., MacLeod A., Tamaki K., Neil D. L., Monckton D. G. Minisatellite repeat coding as a digital approach to DNA typing. Nature. 1991 Nov 21;354(6350):204–209. doi: 10.1038/354204a0. [DOI] [PubMed] [Google Scholar]
- Kerem B. S., Zielenski J., Markiewicz D., Bozon D., Gazit E., Yahav J., Kennedy D., Riordan J. R., Collins F. S., Rommens J. M. Identification of mutations in regions corresponding to the two putative nucleotide (ATP)-binding folds of the cystic fibrosis gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8447–8451. doi: 10.1073/pnas.87.21.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Knowles M. R., Boucher R. C., O'Brien W. E., Beaudet A. L. Benign missense variations in the cystic fibrosis gene. Am J Hum Genet. 1990 Oct;47(4):611–615. [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Kellogg D. E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J. J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990 Feb 25;18(4):999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lench N., Stanier P., Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988 Jun 18;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Main B. F., Jones P. J., MacGillivray R. T., Banfield D. K. Apolipoprotein E genotyping using the polymerase chain reaction and allele-specific oligonucleotide primers. J Lipid Res. 1991 Jan;32(1):183–187. [PubMed] [Google Scholar]
- Mullis K. B. The polymerase chain reaction in an anemic mode: how to avoid cold oligodeoxyribonuclear fusion. PCR Methods Appl. 1991 Aug;1(1):1–4. doi: 10.1101/gr.1.1.1. [DOI] [PubMed] [Google Scholar]
- Myerowitz R. Splice junction mutation in some Ashkenazi Jews with Tay-Sachs disease: evidence against a single defect within this ethnic group. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3955–3959. doi: 10.1073/pnas.85.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old J. M., Varawalla N. Y., Weatherall D. J. Rapid detection and prenatal diagnosis of beta-thalassaemia: studies in Indian and Cypriot populations in the UK. Lancet. 1990 Oct 6;336(8719):834–837. doi: 10.1016/0140-6736(90)92338-i. [DOI] [PubMed] [Google Scholar]
- Osborne L., Knight R., Santis G., Hodson M. A mutation in the second nucleotide binding fold of the cystic fibrosis gene. Am J Hum Genet. 1991 Mar;48(3):608–612. [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Rommens J., Kerem B. S., Greer W., Chang P., Tsui L. C., Ray P. Rapid nonradioactive detection of the major cystic fibrosis mutation. Am J Hum Genet. 1990 Feb;46(2):395–396. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Cassady J., Bottema C. D., Sommer S. S. Characterization of polymerase chain reaction amplification of specific alleles. Anal Biochem. 1990 Apr;186(1):64–68. doi: 10.1016/0003-2697(90)90573-r. [DOI] [PubMed] [Google Scholar]
- Sommer S. S., Cassady J. D., Sobell J. L., Bottema C. D. A novel method for detecting point mutations or polymorphisms and its application to population screening for carriers of phenylketonuria. Mayo Clin Proc. 1989 Nov;64(11):1361–1372. doi: 10.1016/s0025-6196(12)65378-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vidaud M., Fanen P., Martin J., Ghanem N., Nicolas S., Goossens M. Three point mutations in the CFTR gene in French cystic fibrosis patients: identification by denaturing gradient gel electrophoresis. Hum Genet. 1990 Sep;85(4):446–449. doi: 10.1007/BF02428305. [DOI] [PubMed] [Google Scholar]
- Wagner M., Schloesser M., Reiss J. Direct gene diagnosis of cystic fibrosis by allele-specific polymerase chain reactions. Mol Biol Med. 1990 Aug;7(4):359–364. [PubMed] [Google Scholar]
- Wenham P. R., Newton C. R., Price W. H. Analysis of apolipoprotein E genotypes by the Amplification Refractory Mutation System. Clin Chem. 1991 Feb;37(2):241–244. [PubMed] [Google Scholar]
- Wu D. Y., Ugozzoli L., Pal B. K., Wallace R. B. Allele-specific enzymatic amplification of beta-globin genomic DNA for diagnosis of sickle cell anemia. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2757–2760. doi: 10.1073/pnas.86.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielenski J., Bozon D., Kerem B., Markiewicz D., Durie P., Rommens J. M., Tsui L. C. Identification of mutations in exons 1 through 8 of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics. 1991 May;10(1):229–235. doi: 10.1016/0888-7543(91)90504-8. [DOI] [PubMed] [Google Scholar]