Abstract
Models are developed for the survival, history, and spread of variant alleles, in order to consider what can, and what cannot, be inferred from this type of data. The high variances of the processes involved, and questions of sampling, place severe limitations on inferences. Nonetheless, by combining information on a number of rare variants observed in a group of interrelated populations, reliable qualitative inferences are possible. These ideas and models are developed in the context of data on five rare variants and six private polymorphisms observed in eight Chibcha-speaking tribes of Costa Rica and Panama. The decline and fragmentation of the Amerindian populations of Central America over the last 300 years create considerable difficulties in attempting inference of past genetic events. However, these tribes have been well studied genetically, anthropologically, and linguistically and thus provide an excellent framework for the study of rare-variant spread.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Boehm C. D., Serjeant G. R., Theisen C. E., Dover G. J., Kazazian H. H., Jr Origin of the beta S-globin gene in blacks: the contribution of recurrent mutation or gene conversion or both. Proc Natl Acad Sci U S A. 1984 Feb;81(3):853–856. doi: 10.1073/pnas.81.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Orkin S. H., Kazazian H. H., Jr, Goff S. C., Boehm C. D., Waber P. G., Sexton J. P., Ostrer H., Fairbanks V. F., Chakravarti A. Evidence for multiple origins of the beta E-globin gene in Southeast Asia. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6608–6611. doi: 10.1073/pnas.79.21.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnason A., Larsen B., Marshall W. H., Edwards J. H., Macintosh P., Olaisen B., Teisberg P. Very close linkage between HLA-B and Bf inferred from allelic association. Nature. 1977 Aug 11;268(5620):527–528. doi: 10.1038/268527a0. [DOI] [PubMed] [Google Scholar]
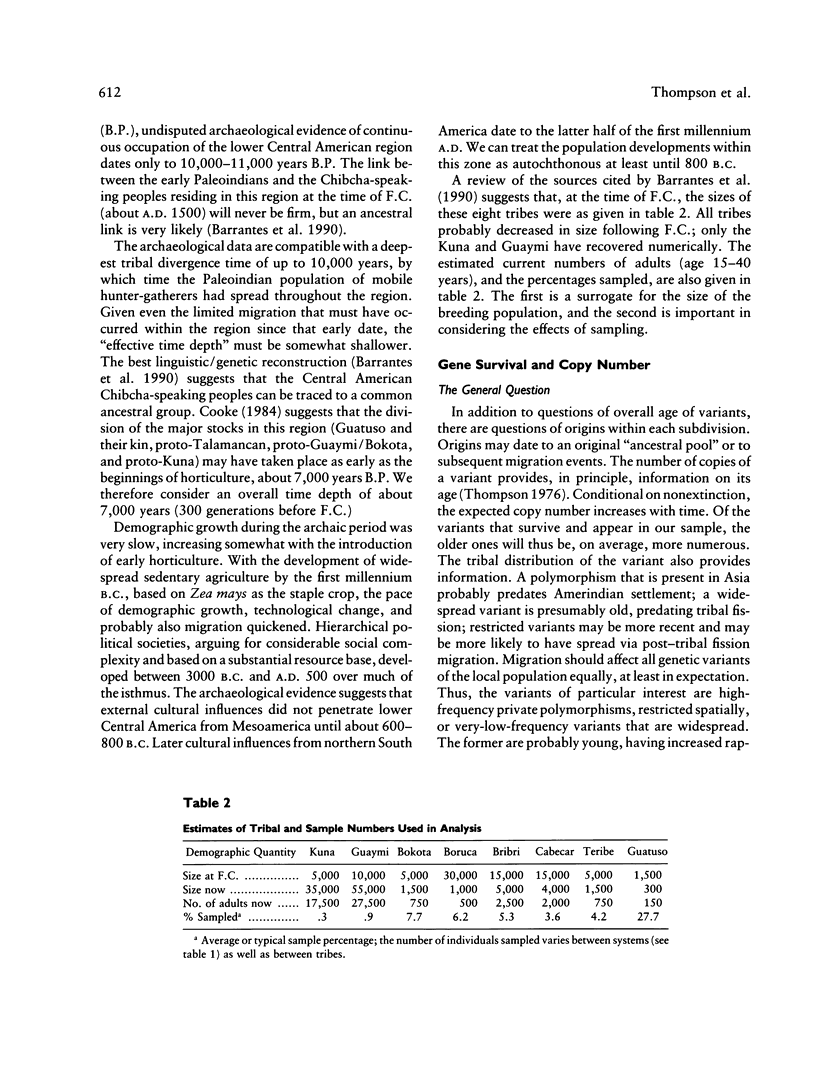
- Barrantes R., Smouse P. E., Mohrenweiser H. W., Gershowitz H., Azofeifa J., Arias T. D., Neel J. V. Microevolution in lower Central America: genetic characterization of the Chibcha-speaking groups of Costa Rica and Panama, and a consensus taxonomy based on genetic and linguistic affinity. Am J Hum Genet. 1990 Jan;46(1):63–84. [PMC free article] [PubMed] [Google Scholar]
- Barrantes R., Smouse P. E., Neel J. V., Mohrenweiser H. W., Gershowitz H. Migration and genetic infrastructure of the Central American Guaymi and their affinities with other tribal groups. Am J Phys Anthropol. 1982 Jun;58(2):201–214. doi: 10.1002/ajpa.1330580213. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Smouse P. E., Neel J. V. Population amalgamation and genetic variation: observations on artificially agglomerated tribal populations of Central and South America. Am J Hum Genet. 1988 Nov;43(5):709–725. [PMC free article] [PubMed] [Google Scholar]
- Chebloune Y., Pagnier J., Trabuchet G., Faure C., Verdier G., Labie D., Nigon V. Structural analysis of the 5' flanking region of the beta-globin gene in African sickle cell anemia patients: further evidence for three origins of the sickle cell mutation in Africa. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4431–4435. doi: 10.1073/pnas.85.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens W. J. The sampling theory of selectively neutral alleles. Theor Popul Biol. 1972 Mar;3(1):87–112. doi: 10.1016/0040-5809(72)90035-4. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. The rate of loss of multiple alleles in finite haploid populations. Theor Popul Biol. 1971 Dec;2(4):391–403. doi: 10.1016/0040-5809(71)90028-1. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Novotny J. E. An enzymatically inactive variant of human lactate dehydrogenase-LDHBGUA-1. Study of subunit interaction. Biochim Biophys Acta. 1982 Mar 18;702(1):90–98. doi: 10.1016/0167-4838(82)90030-9. [DOI] [PubMed] [Google Scholar]
- Neel J. V. Rare variants, private polymorphisms, and locus heterozygosity in Amerindian populations. Am J Hum Genet. 1978 Sep;30(5):465–490. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Smouse P., Asakawa J., Takahashi N., Goriki K., Fujita M., Kageoka T., Hazama R. Protein variants in Hiroshima and Nagasaki: tales of two cities. Am J Hum Genet. 1988 Dec;43(6):870–893. [PMC free article] [PubMed] [Google Scholar]
- Tanis R. J., Neel J. V., Torres de Arauz R. Two more "private" polymorphisms of Amerindian tribes: LDHb GUA-1 and ACP1 B GUA-1 in the Guaymi in Panama. Am J Hum Genet. 1977 Sep;29(5):419–430. [PMC free article] [PubMed] [Google Scholar]
- Thompson E. A. Estimation of age and rate of increase of rare variants. Am J Hum Genet. 1976 Sep;28(5):442–452. [PMC free article] [PubMed] [Google Scholar]
- Thompson E. A., Neel J. V. Probability of founder effect in a tribal population. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1442–1445. doi: 10.1073/pnas.75.3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W. J., Li W. H., Posner I., Yamamura T., Yamamoto A., Gotto A. M., Jr, Chan L. No severe bottleneck during human evolution: evidence from two apolipoprotein C-II deficiency alleles. Am J Hum Genet. 1991 Feb;48(2):383–389. [PMC free article] [PubMed] [Google Scholar]


