Abstract
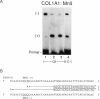
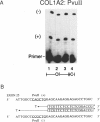
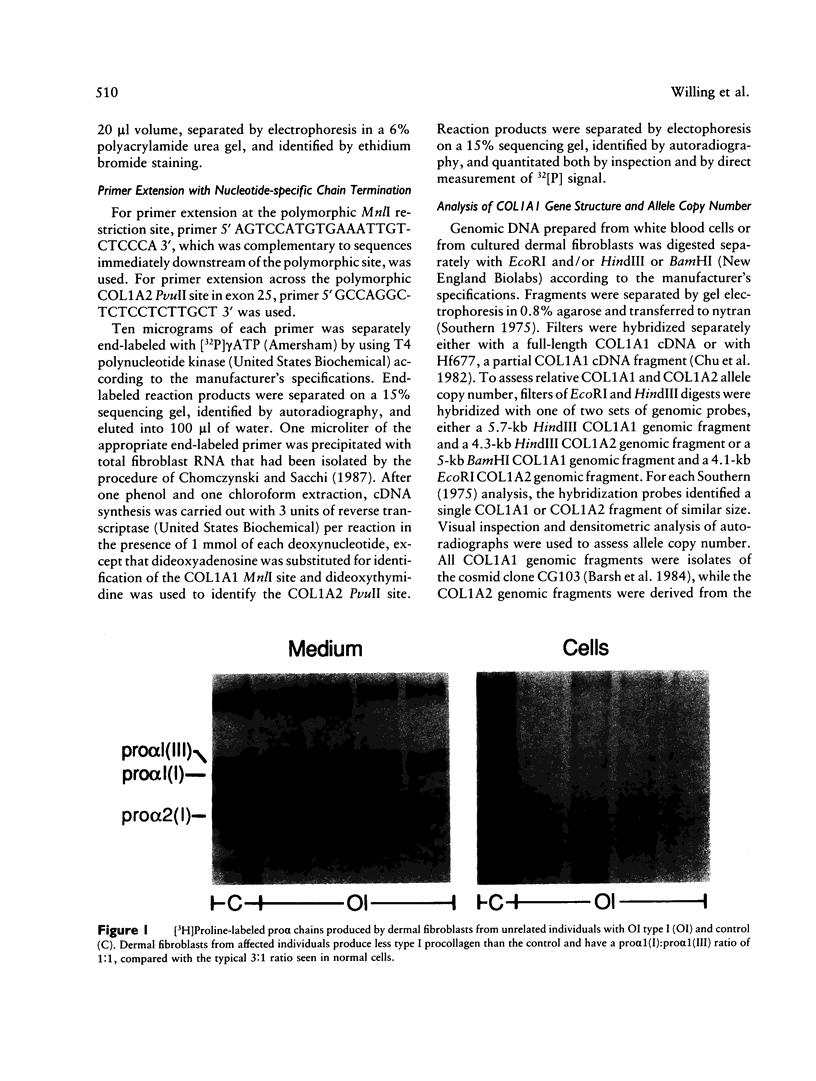
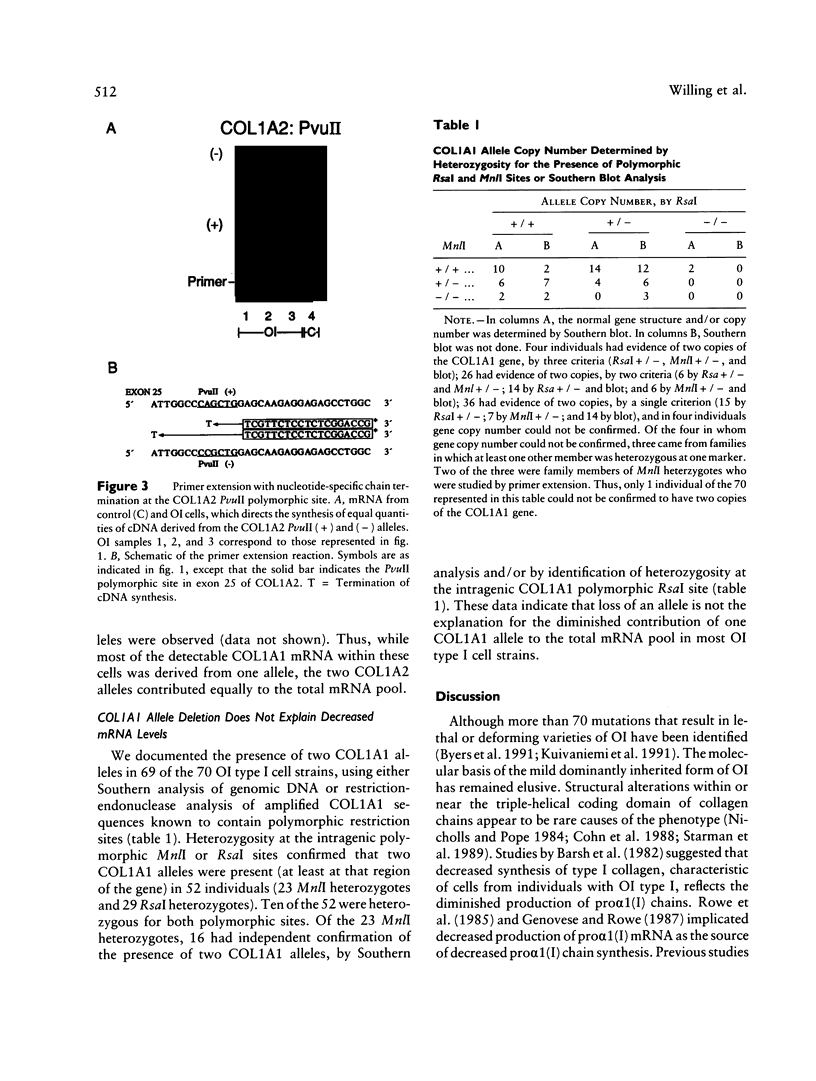
Dermal fibroblasts from most individuals with osteogenesis imperfecta (OI) type I produce about half the normal amount of type I procollagen, as a result of decreased synthesis of one of its constituent chains, pro alpha 1 (I). To test the hypothesis that decreased synthesis of pro alpha (I) chains results from mutations in the COL1A1 gene, we used primer extension with nucleotide-specific chain termination to measure the contribution of individual COL1A1 alleles to the mRNA pool in fibroblasts from affected individuals. A polymorphic MnlI restriction endonuclease site in the 3'-untranslated region of COL1A1 was used to distinguish the transcripts of the two alleles in heterozygous individuals. Twenty-three individuals from 21 unrelated families were studied. In each case there was marked diminution in steady-state mRNA levels from one COL1A1 allele. Loss of an allele through deletion or rearrangement was not the cause of the diminished COL1A1 mRNA levels. Primer extension with nucleotide-specific chain termination allows identification of the mutant COL1A1 allele in cell strains that are heterozygous for an expressed polymorphism. It is applicable to sporadic cases, to small families, and to large families in whom key individuals are uninformative at the polymorphic sites used in linkage analysis, making it a useful adjunct to the biochemical screening of collagenous proteins for OI.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Gelinas R. E. DNA and chromatin structure of the human alpha 1 (I) collagen gene. J Biol Chem. 1984 Dec 10;259(23):14906–14913. [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Bonadio J., Saunders T. L., Tsai E., Goldstein S. A., Morris-Wiman J., Brinkley L., Dolan D. F., Altschuler R. A., Hawkins J. E., Jr, Bateman J. F. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7145–7149. doi: 10.1073/pnas.87.18.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Shapiro J. R., Rowe D. W., David K. E., Holbrook K. A. Abnormal alpha 2-chain in type I collagen from a patient with a form of osteogenesis imperfecta. J Clin Invest. 1983 Mar;71(3):689–697. doi: 10.1172/JCI110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Wallis G. A., Willing M. C. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet. 1991 Jul;28(7):433–442. doi: 10.1136/jmg.28.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Apone S., Eyre D. R., Starman B. J., Andreassen P., Charbonneau H., Nicholls A. C., Pope F. M., Byers P. H. Substitution of cysteine for glycine within the carboxyl-terminal telopeptide of the alpha 1 chain of type I collagen produces mild osteogenesis imperfecta. J Biol Chem. 1988 Oct 15;263(29):14605–14607. [PubMed] [Google Scholar]
- Constantinou C. D., Spotila L. D., Zhuang J., Sereda L., Hanning C., Prockop D. J. PvuII polymorphism at the COL1A2 locus. Nucleic Acids Res. 1990 Sep 25;18(18):5577–5577. doi: 10.1093/nar/18.18.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio M., Bernard M., Pretorius P. J., de Wet W., Ramirez F., Pretorious P. J. Complete nucleotide sequence of the region encompassing the first twenty-five exons of the human pro alpha 1(I) collagen gene (COL1A1) Gene. 1988 Jul 15;67(1):105–115. doi: 10.1016/0378-1119(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Wynne J. K., Wallis S. C., Scott J. An in vitro system for the editing of apolipoprotein B mRNA. Cell. 1989 Aug 11;58(3):519–525. doi: 10.1016/0092-8674(89)90432-7. [DOI] [PubMed] [Google Scholar]
- Falk C. T., Schwartz R. C., Ramirez F., Tsipouras P. Use of molecular haplotypes specific for the human pro alpha 2(I) collagen gene in linkage analysis of the mild autosomal dominant forms of osteogenesis imperfecta. Am J Hum Genet. 1986 Mar;38(3):269–279. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Genovese C., Rowe D. Analysis of cytoplasmic and nuclear messenger RNA in fibroblasts from patients with type I osteogenesis imperfecta. Methods Enzymol. 1987;145:223–235. doi: 10.1016/0076-6879(87)45012-x. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Chu M. L., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988 Jun 15;252(3):633–640. doi: 10.1042/bj2520633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., Pope F. M., Craig D. An abnormal collagen alpha chain containing cysteine in autosomal dominant osteogenesis imperfecta. Br Med J (Clin Res Ed) 1984 Jan 14;288(6411):112–113. doi: 10.1136/bmj.288.6411.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruchno C. J., Cohn D. H., Wallis G. A., Willing M. C., Starman B. J., Zhang X. M., Byers P. H. Osteogenesis imperfecta due to recurrent point mutations at CpG dinucleotides in the COL1A1 gene of type I collagen. Hum Genet. 1991 May;87(1):33–40. doi: 10.1007/BF01213088. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Shapiro J. R., Poirier M., Schlesinger S. Diminished type I collagen synthesis and reduced alpha 1(I) collagen messenger RNA in cultured fibroblasts from patients with dominantly inherited (type I) osteogenesis imperfecta. J Clin Invest. 1985 Aug;76(2):604–611. doi: 10.1172/JCI112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Starman B. J., Eyre D., Charbonneau H., Harrylock M., Weis M. A., Weiss L., Graham J. M., Jr, Byers P. H. Osteogenesis imperfecta. The position of substitution for glycine by cysteine in the triple helical domain of the pro alpha 1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest. 1989 Oct;84(4):1206–1214. doi: 10.1172/JCI114286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Francis M. J., Smith R. Altered relation of two collagen types in osteogenesis imperfecta. N Engl J Med. 1977 May 26;296(21):1200–1203. doi: 10.1056/NEJM197705262962104. [DOI] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Anderson, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986 Jul 12;2(8498):69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Wallis G., Mathew C., Beighton P., Nicholls A., Pope F. M., Thompson E., Tsipouras P. Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Hum Genet. 1990 Feb;46(2):293–307. [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Børresen A. L., Dickson L. A., Berg K., Prockop D. J., Ramirez F. Molecular heterogeneity in the mild autosomal dominant forms of osteogenesis imperfecta. Am J Hum Genet. 1984 Nov;36(6):1172–1179. [PMC free article] [PubMed] [Google Scholar]
- Wallis G., Beighton P., Boyd C., Mathew C. G. Mutations linked to the pro alpha 2(I) collagen gene are responsible for several cases of osteogenesis imperfecta type I. J Med Genet. 1986 Oct;23(5):411–416. doi: 10.1136/jmg.23.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup R. J., Willing M. C., Starman B. J., Byers P. H. Distinct biochemical phenotypes predict clinical severity in nonlethal variants of osteogenesis imperfecta. Am J Hum Genet. 1990 May;46(5):975–982. [PMC free article] [PubMed] [Google Scholar]
- Westerhausen A. I., Constantinou C. D., Prockop D. J. A sequence polymorphism in the 3'-nontranslated region of the pro alpha 1 chain of type I procollagen. Nucleic Acids Res. 1990 Aug 25;18(16):4968–4968. doi: 10.1093/nar/18.16.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Byers P. H. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990 Jan;85(1):282–290. doi: 10.1172/JCI114424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Starman B., Holbrook K. A., Greenberg C. R., Byers P. H. Heterozygosity for a large deletion in the alpha 2(I) collagen gene has a dramatic effect on type I collagen secretion and produces perinatal lethal osteogenesis imperfecta. J Biol Chem. 1988 Jun 15;263(17):8398–8404. [PubMed] [Google Scholar]