Abstract
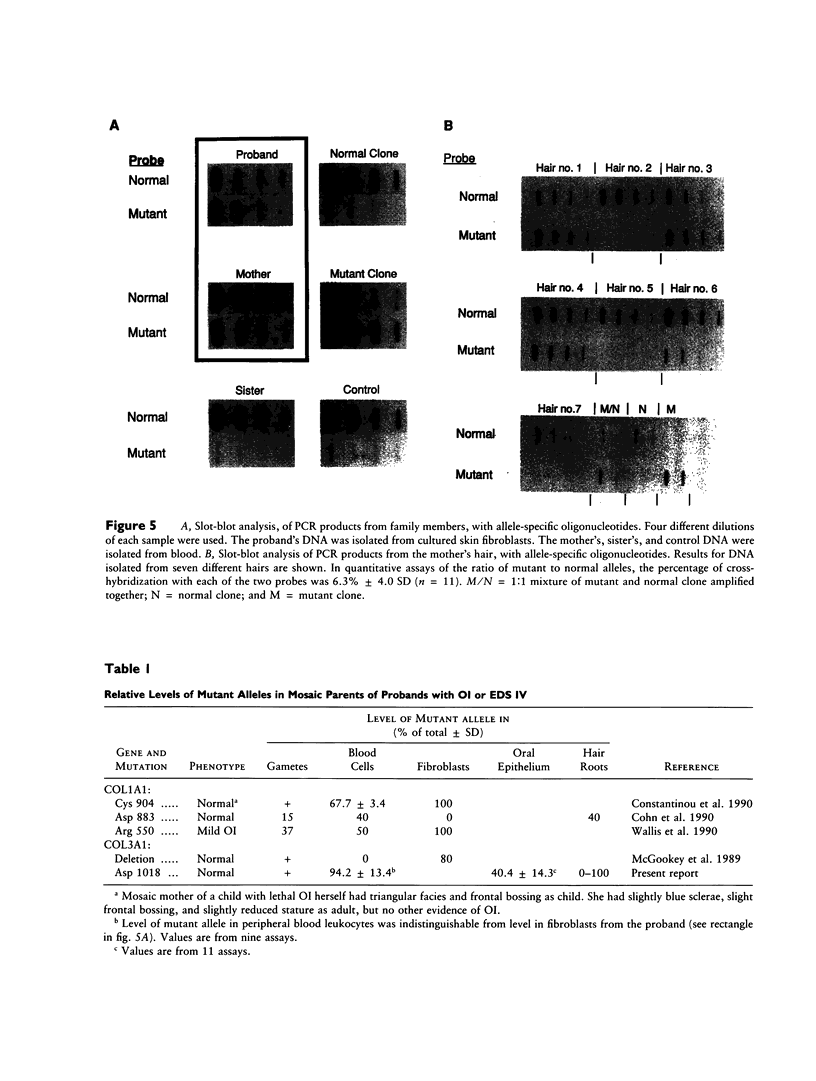
A proband with arterial ruptures and skin changes characteristic of the type IV variant of Ehlers-Danlos syndrome was found to have a single-base mutation in the type III procollagen gene, which converted the codon for glycine at amino acid position 1018 to a codon for aspartate. (Amino acid positions are numbered by the standard convention in which the first glycine of the triple-helical domain of an alpha chain is number 1. The numbers of positions in the alpha 1(III) chains can be converted to positions in the human pro alpha(III) chain by adding 167.) Nucleotide sequencing of overlapping PCR products in which the two alleles were distinguished demonstrated that the mutation of glycine 1018 was the only mutation that changed the primary structure of type III procollagen. The glycine substitution markedly decreased the amount of type III procollagen secreted into the medium by cultured skin fibroblasts from the proband. It is surprising that the same mutation was found in about 94% of the peripheral blood leukocytes from the proband's asymptomatic 72-year-old mother. Other tissues from the mother contained the mutated allele; it was present in 0%-100% of different samples of hair cells and in about 40% of cells from the oral epithelium. Therefore, the mother was a mosaic for the mutation. Since the mutated allele was present in cells derived from all three germ layers, the results indicated that the mutation arose by the late blastocyst stage of development. The results also indicate that assays of blood leukocytes do not always reveal mosaicism or predict phenotypic involvement of tissues, such as blood vessels, that are derived from the same embryonic cells as are leukocytes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Kontusaari S., Baldwin C. T., Kuivaniemi H., Prockop D. J. Structure of cDNA clones coding for the entire prepro alpha 1 (III) chain of human type III procollagen. Differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J. 1989 Jun 1;260(2):509–516. doi: 10.1042/bj2600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E., Van Broeckhoven C., Bonten E. J., van de Vooren M. J., Veenema H., Van Hul W., Van Ommen G. J., Vandenberghe A., Pearson P. L. Germline mosaicism and Duchenne muscular dystrophy mutations. Nature. 1987 Oct 8;329(6139):554–556. doi: 10.1038/329554a0. [DOI] [PubMed] [Google Scholar]
- Bakker E., Veenema H., Den Dunnen J. T., van Broeckhoven C., Grootscholten P. M., Bonten E. J., van Ommen G. J., Pearson P. L. Germinal mosaicism increases the recurrence risk for 'new' Duchenne muscular dystrophy mutations. J Med Genet. 1989 Sep;26(9):553–559. doi: 10.1136/jmg.26.9.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
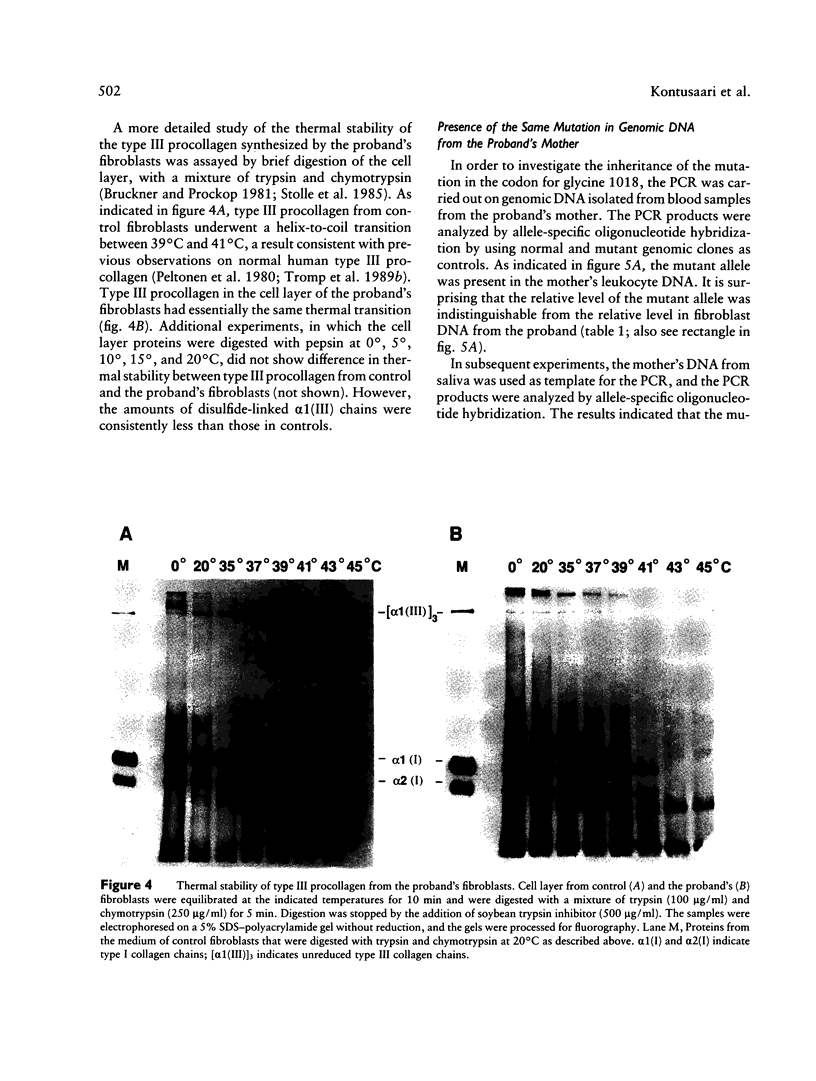
- Byers P. H., Holbrook K. A., Barsh G. S., Smith L. T., Bornstein P. Altered secretion of type III procollagen in a form of type IV Ehlers-Danlos syndrome. Biochemical studies in cultured fibroblasts. Lab Invest. 1981 Apr;44(4):336–341. [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Weil D., de Wet W., Bernard M., Sippola M., Ramirez F. Isolation of cDNA and genomic clones encoding human pro-alpha 1 (III) collagen. Partial characterization of the 3' end region of the gene. J Biol Chem. 1985 Apr 10;260(7):4357–4363. [PubMed] [Google Scholar]
- Cohn D. H., Starman B. J., Blumberg B., Byers P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet. 1990 Mar;46(3):591–601. [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chiodo A. A., Lamande S. R., Janeczko R., Ramirez F., Dahl H. H., Chan D., Bateman J. F. A base substitution at a splice site in the COL3A1 gene causes exon skipping and generates abnormal type III procollagen in a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1990 Oct 5;265(28):17070–17077. [PubMed] [Google Scholar]
- Constantinou C. D., Pack M., Young S. B., Prockop D. J. Phenotypic heterogeneity in osteogenesis imperfecta: the mildly affected mother of a proband with a lethal variant has the same mutation substituting cysteine for alpha 1-glycine 904 in a type I procollagen gene (COL1A1). Am J Hum Genet. 1990 Oct;47(4):670–679. [PMC free article] [PubMed] [Google Scholar]
- Darras B. T., Francke U. A partial deletion of the muscular dystrophy gene transmitted twice by an unaffected male. Nature. 1987 Oct 8;329(6139):556–558. doi: 10.1038/329556a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Ladda R. L., Prockop D. J. Inheritance of an RNA splicing mutation (G+ 1 IVS20) in the type III procollagen gene (COL3A1) in a family having aortic aneurysms and easy bruisability: phenotypic overlap between familial arterial aneurysms and Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1990 Jul;47(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Romanic A. M., Prockop D. J. A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest. 1990 Nov;86(5):1465–1473. doi: 10.1172/JCI114863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Kontusaari S., Tromp G., Zhao M. J., Sabol C., Prockop D. J. Identical G+1 to A mutations in three different introns of the type III procollagen gene (COL3A1) produce different patterns of RNA splicing in three variants of Ehlers-Danlos syndrome. IV. An explanation for exon skipping some mutations and not others. J Biol Chem. 1990 Jul 15;265(20):12067–12074. [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Lee B., D'Alessio M., Vissing H., Ramirez F., Steinmann B., Superti-Furga A. Characterization of a large deletion associated with a polymorphic block of repeated dinucleotides in the type III procollagen gene (COL3A1) of a patient with Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1991 Mar;48(3):511–517. [PMC free article] [PubMed] [Google Scholar]
- Lee B., Vitale E., Superti-Furga A., Steinmann B., Ramirez F. G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5256–5259. [PubMed] [Google Scholar]
- Loidl H. R., Brinker J. M., May M., Pihlajaniemi T., Morrow S., Rosenbloom J., Myers J. C. Molecular cloning and carboxyl-propeptide analysis of human type III procollagen. Nucleic Acids Res. 1984 Dec 21;12(24):9383–9394. doi: 10.1093/nar/12.24.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankoo B. S., Dalgleish R. Human pro alpha 1(III) collagen: cDNA sequence for the 3' end. Nucleic Acids Res. 1988 Mar 25;16(5):2337–2337. doi: 10.1093/nar/16.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskulin M., Dalgleish R., Kluve-Beckerman B., Rennard S. I., Tolstoshev P., Brantly M., Crystal R. G. Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochemistry. 1986 Mar 25;25(6):1408–1413. doi: 10.1021/bi00354a033. [DOI] [PubMed] [Google Scholar]
- Morris D. Acrogeria. Proc R Soc Med. 1957 May;50(5):330–331. doi: 10.1177/003591575705000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil-Dwyer G., Bartlett J. R., Nicholls A. C., Narcisi P., Pope F. M. Collagen deficiency and ruptured cerebral aneurysms. A clinical and biochemical study. J Neurosurg. 1983 Jul;59(1):16–20. doi: 10.3171/jns.1983.59.1.0016. [DOI] [PubMed] [Google Scholar]
- Ostergaard J. R., Oxlund H. Collagen type III deficiency in patients with rupture of intracranial saccular aneurysms. J Neurosurg. 1987 Nov;67(5):690–696. doi: 10.3171/jns.1987.67.5.0690. [DOI] [PubMed] [Google Scholar]
- Peltonen L., Palotie A., Hayashi T., Prockop D. J. Thermal stability of type I and type III procollagens from normal human fibroblasts and from a patient with osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1980 Jan;77(1):162–166. doi: 10.1073/pnas.77.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope F. M., Child A. H., Nicholls A. C., Narcisi P., Dorrance D. E. Type III collagen deficiency with normal phenotype. J R Soc Med. 1983 Jun;76(6):518–520. [PMC free article] [PubMed] [Google Scholar]
- Pope F. M., Martin G. R., Lichtenstein J. R., Penttinen R., Gerson B., Rowe D. W., McKusick V. A. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. J., Lloyd J. C., Ward P. N., De Paepe A., Narcisi P., Pope F. M. Characterisation of a glycine to valine substitution at amino acid position 910 of the triple helical region of type III collagen in a patient with Ehlers-Danlos syndrome type IV. J Med Genet. 1991 Jul;28(7):458–463. doi: 10.1136/jmg.28.7.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Stolle C. A., Pyeritz R. E., Myers J. C., Prockop D. J. Synthesis of an altered type III procollagen in a patient with type IV Ehlers-Danlos syndrome. A structural change in the alpha 1(III) chain which makes the protein more susceptible to proteinases. J Biol Chem. 1985 Feb 10;260(3):1937–1944. [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Steinmann B., Ramirez F., Byers P. H. Molecular defects of type III procollagen in Ehlers-Danlos syndrome type IV. Hum Genet. 1989 May;82(2):104–108. doi: 10.1007/BF00284038. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Toman P. D., Ricca G. A., de Crombrugghe B. Nucleotide sequence of a cDNA coding for the amino-terminal region of human prepro alpha 1(III) collagen. Nucleic Acids Res. 1988 Jul 25;16(14B):7201–7201. doi: 10.1093/nar/16.14.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Shikata H., Prockop D. J. A single base mutation that substitutes serine for glycine 790 of the alpha 1 (III) chain of type III procollagen exposes an arginine and causes Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Jan 25;264(3):1349–1352. [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stolle C., Pope F. M., Prockop D. J. Single base mutation in the type III procollagen gene that converts the codon for glycine 883 to aspartate in a mild variant of Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Nov 15;264(32):19313–19317. [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Byers P. H., Steinmann B., Superti-Furga A. Multiexon deletion in the procollagen III gene is associated with mild Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5244–5248. [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E. Dinucleotide repeat polymorphisms at the D7S435 and D7S440 loci. Nucleic Acids Res. 1990 Jul 11;18(13):4039–4039. doi: 10.1093/nar/18.13.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Dinucleotide repeat polymorphism at the D18S34 locus. Nucleic Acids Res. 1990 Apr 25;18(8):2201–2201. [PMC free article] [PubMed] [Google Scholar]
- Zafarullah K., Kleinert C., Tromp G., Kuivaniemi H., Kontusaari S., Wu Y. L., Ganguly A., Prockop D. J. G to A polymorphism in exon 31 of the COL3A1 gene. Nucleic Acids Res. 1990 Oct 25;18(20):6180–6180. doi: 10.1093/nar/18.20.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paepe A., van Landegem W., de Keyser F., de Reuck J. Association of multiple intracranial aneurysms and collagen type III deficiency. Clin Neurol Neurosurg. 1988;90(1):53–56. doi: 10.1016/s0303-8467(88)80010-6. [DOI] [PubMed] [Google Scholar]