Abstract
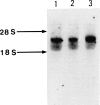
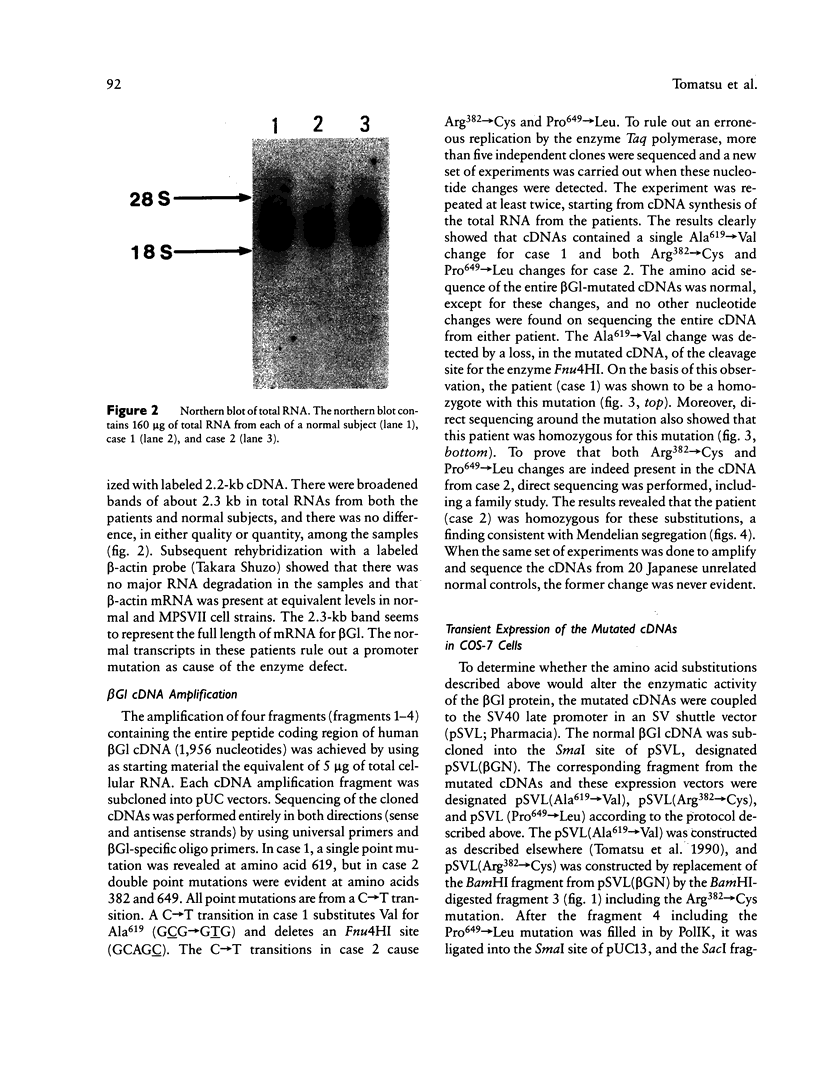
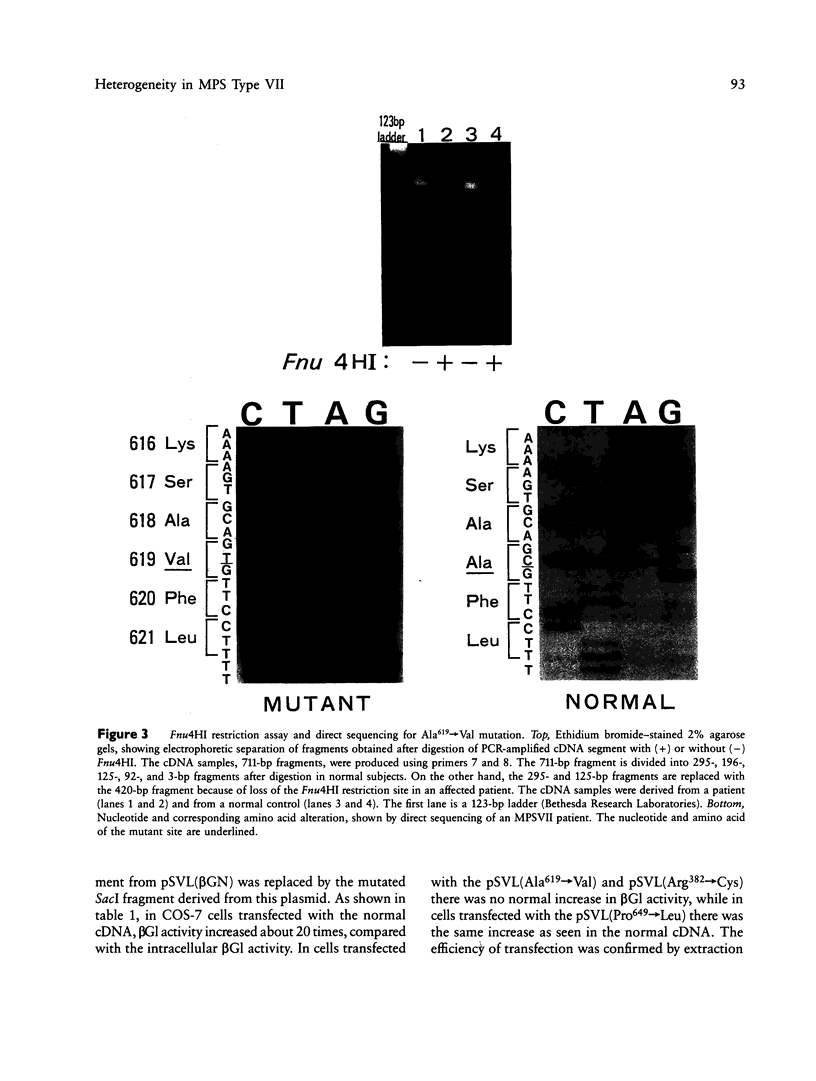
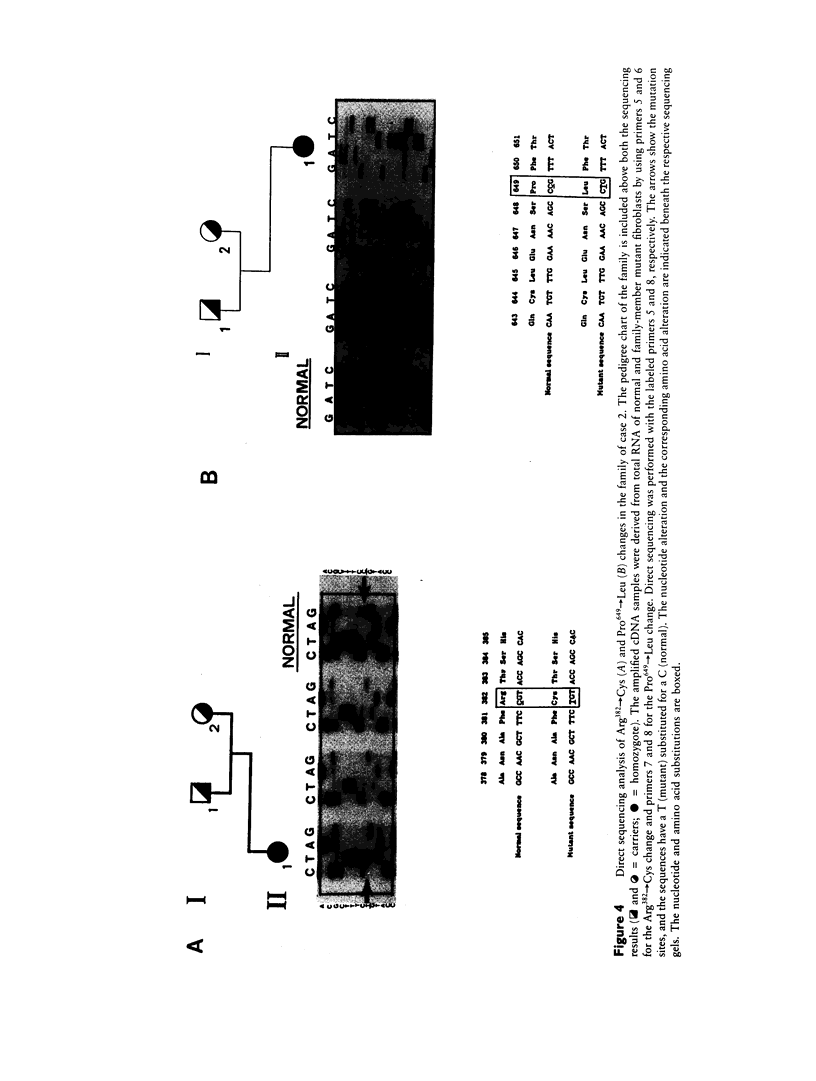
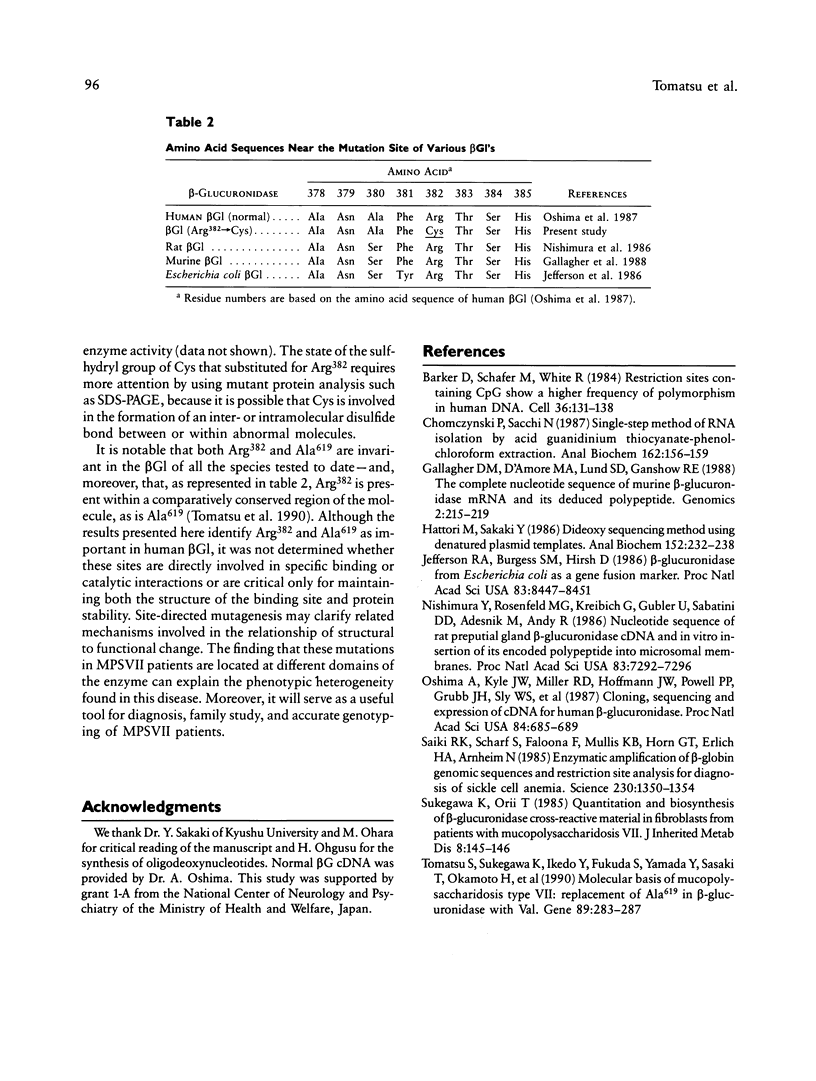
We identified two different exonic point mutations causing β-glucuronidase (βGl) deficiency in two Japanese patients with mucopolysaccharidosis type VII (MPSVII). Enzyme assay of lysates of the lymphocytes and cultured fibroblasts showed little residual activity. The βGl-specific mRNA levels were normal, as determined by northern blot analysis. Mutated cDNA clones, including the entire coding sequence, were isolated using the polymerase chain reaction (PCR) products derived from βGl-deficient fibroblasts. Sequence analysis of the full-length mutated cDNAs showed C→T transitions, which resulted in a single Ala619→Val change (case 1, a 24-year-old male) and a Arg382→Cys change (case 2, a 7-year-old female). The former change was revealed by a loss of the cleavage site for the Fnu4HI in the mutated cDNA. On the basis of the loss of Fnu4HI restriction site, the patient (case 1) was a homozygote with this mutation. The mutational change in patient 2 was confirmed by direct sequencing and by demonstrating heterozygosity for the mutation in her parents. The Ala619→Val and Arg382→Cys mutations each disrupt a different domain which is highly conserved among human, rat, and Escherichia coli βGls. Each of these two amino acid changes reduced the βGl activity of the corresponding mutant βGl expressed following transfection of COS cells with expression vectors harboring the mutated cDNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Gallagher P. M., D'Amore M. A., Lund S. D., Ganschow R. E. The complete nucleotide sequence of murine beta-glucuronidase mRNA and its deduced polypeptide. Genomics. 1988 Apr;2(3):215–219. doi: 10.1016/0888-7543(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Rosenfeld M. G., Kreibich G., Gubler U., Sabatini D. D., Adesnik M., Andy R. Nucleotide sequence of rat preputial gland beta-glucuronidase cDNA and in vitro insertion of its encoded polypeptide into microsomal membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7292–7296. doi: 10.1073/pnas.83.19.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A., Kyle J. W., Miller R. D., Hoffmann J. W., Powell P. P., Grubb J. H., Sly W. S., Tropak M., Guise K. S., Gravel R. A. Cloning, sequencing, and expression of cDNA for human beta-glucuronidase. Proc Natl Acad Sci U S A. 1987 Feb;84(3):685–689. doi: 10.1073/pnas.84.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sukegawa K., Orii T. Quantitation and biosynthesis of beta-glucuronidase cross-reactive material in fibroblasts from patients with mucopolysaccharidosis VII. J Inherit Metab Dis. 1985;8(3):145–146. doi: 10.1007/BF01819300. [DOI] [PubMed] [Google Scholar]
- Tomatsu S., Sukegawa K., Ikedo Y., Fukuda S., Yamada Y., Sasaki T., Okamoto H., Kuwabara T., Orii T. Molecular basis of mucopolysaccharidosis type VII: replacement of Ala619 in beta-glucuronidase with Val. Gene. 1990 May 14;89(2):283–287. doi: 10.1016/0378-1119(90)90019-n. [DOI] [PubMed] [Google Scholar]