Abstract
Allelic data for the D1S80 locus was obtained by using the PCR and subsequent analysis with a high-resolution, horizontal PAGE technique and silver staining. Compared with RFLP analysis of VNTR loci by Southern blotting, the approach described in this paper offers certain advantages: (1) discrete allele resolution, (2) minimal measurement error, (3) correct genotyping of single-band VNTR patterns, (4) a nonisotopic assay, (5) a permanent record of the electrophoretic separation, and (6) reduced assay time. In a sample of 99 unrelated Caucasians, the D1S80 locus demonstrated a heterozygosity of 80.8% with 37 phenotypes and 16 alleles. The distribution of genotypes is in agreement with expected values according to the Hardy-Weinberg equilibrium. Furthermore, the observed number of alleles and the level of heterozygosity, obtained through the protocol described here, were congruent with each other in accordance with the expectation of a mutation-drift equilibrium model for a single, homogeneous, random-mating population. Therefore, the analysis of D1S80 and similar VNTR loci by amplified fragment length polymorphism (AMP-FLP) may prove useful as models for population genetic issues for VNTR loci analyzed by RFLP typing via Southern blotting.
Full text
PDF


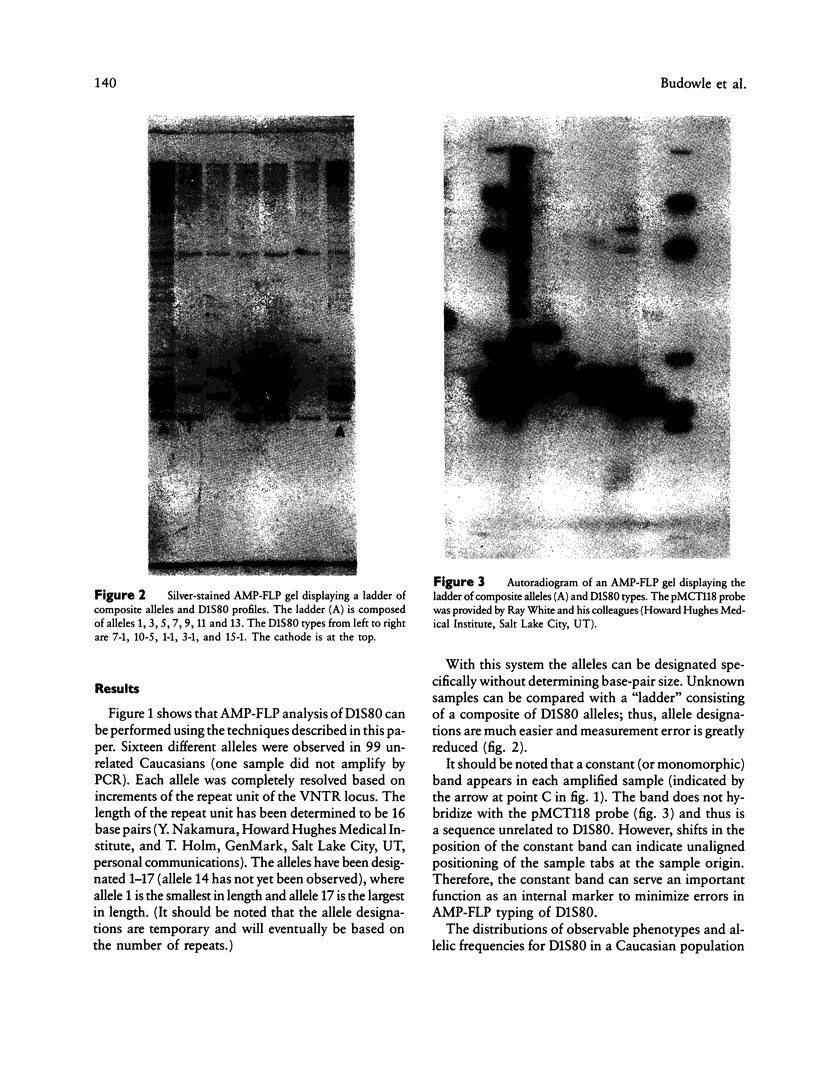




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Graves G., Budowle B. Polymerase chain reaction amplification products separated on rehydratable polyacrylamide gels and stained with silver. Biotechniques. 1989 Jul-Aug;7(7):736–744. [PubMed] [Google Scholar]
- Boerwinkle E., Xiong W. J., Fourest E., Chan L. Rapid typing of tandemly repeated hypervariable loci by the polymerase chain reaction: application to the apolipoprotein B 3' hypervariable region. Proc Natl Acad Sci U S A. 1989 Jan;86(1):212–216. doi: 10.1073/pnas.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budowle B., Baechtel F. S. Modifications to improve the effectiveness of restriction fragment length polymorphism typing. Appl Theor Electrophor. 1990;1(4):181–187. [PubMed] [Google Scholar]
- Chakraborty R. Mitochondrial DNA polymorphism reveals hidden heterogeneity within some Asian populations. Am J Hum Genet. 1990 Jul;47(1):87–94. [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R., Smouse P. E., Neel J. V. Population amalgamation and genetic variation: observations on artificially agglomerated tribal populations of Central and South America. Am J Hum Genet. 1988 Nov;43(5):709–725. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Horn G. T., Richards B., Klinger K. W. Amplification of a highly polymorphic VNTR segment by the polymerase chain reaction. Nucleic Acids Res. 1989 Mar 11;17(5):2140–2140. doi: 10.1093/nar/17.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig E. H., Friedl W., McCarthy B. J. High-resolution analysis of a hypervariable region in the human apolipoprotein B gene. Am J Hum Genet. 1989 Sep;45(3):458–464. [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Carlson M., Krapcho K., White R. Isolation and mapping of a polymorphic DNA sequence (pMCT118) on chromosome 1p [D1S80]. Nucleic Acids Res. 1988 Oct 11;16(19):9364–9364. doi: 10.1093/nar/16.19.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]






