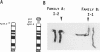Abstract
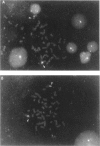
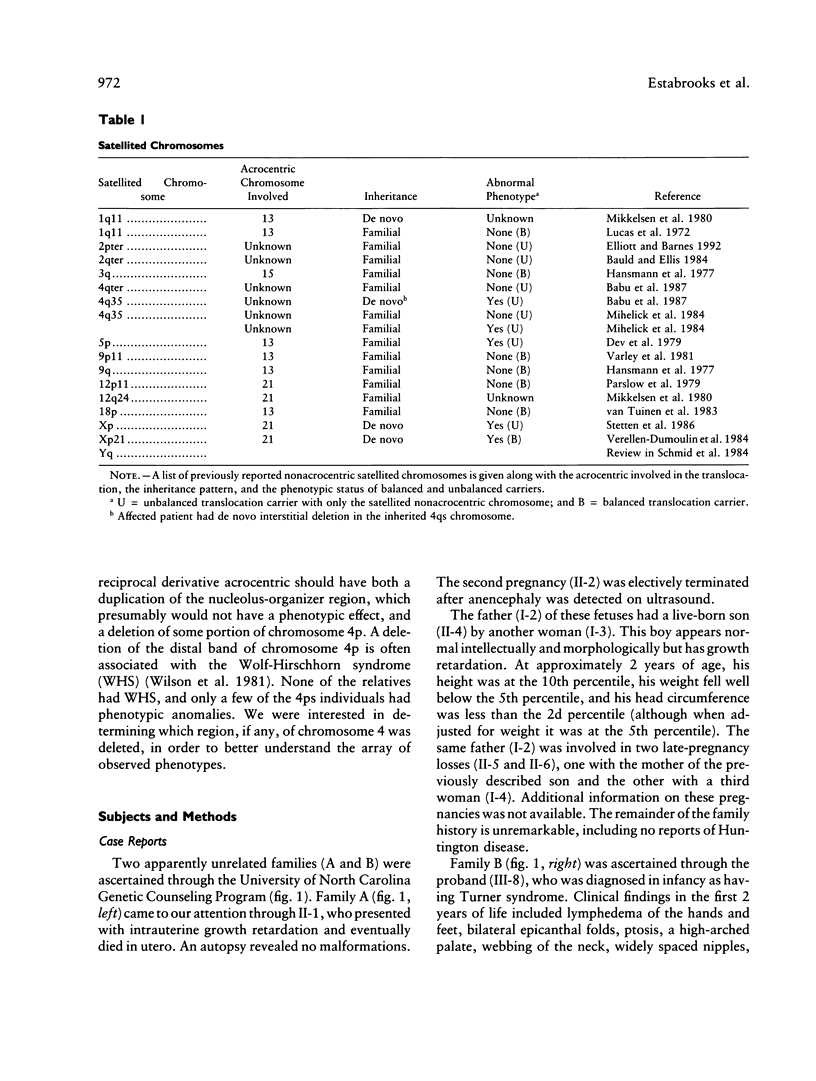
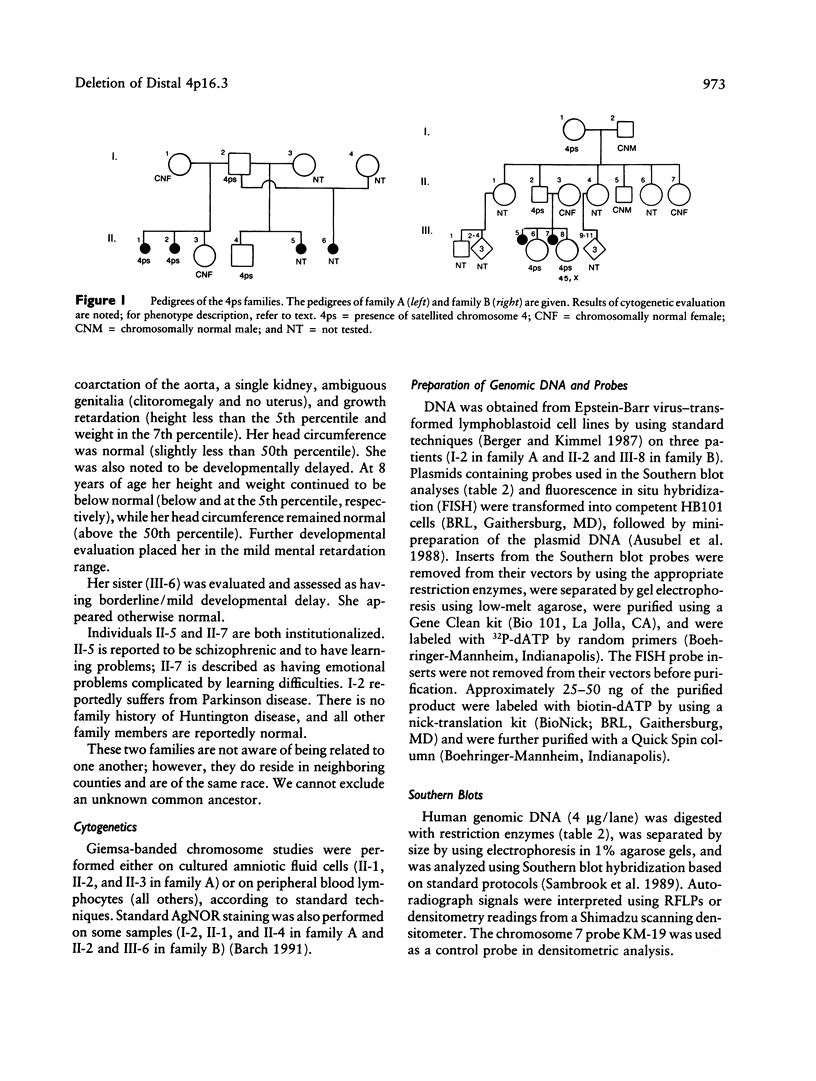
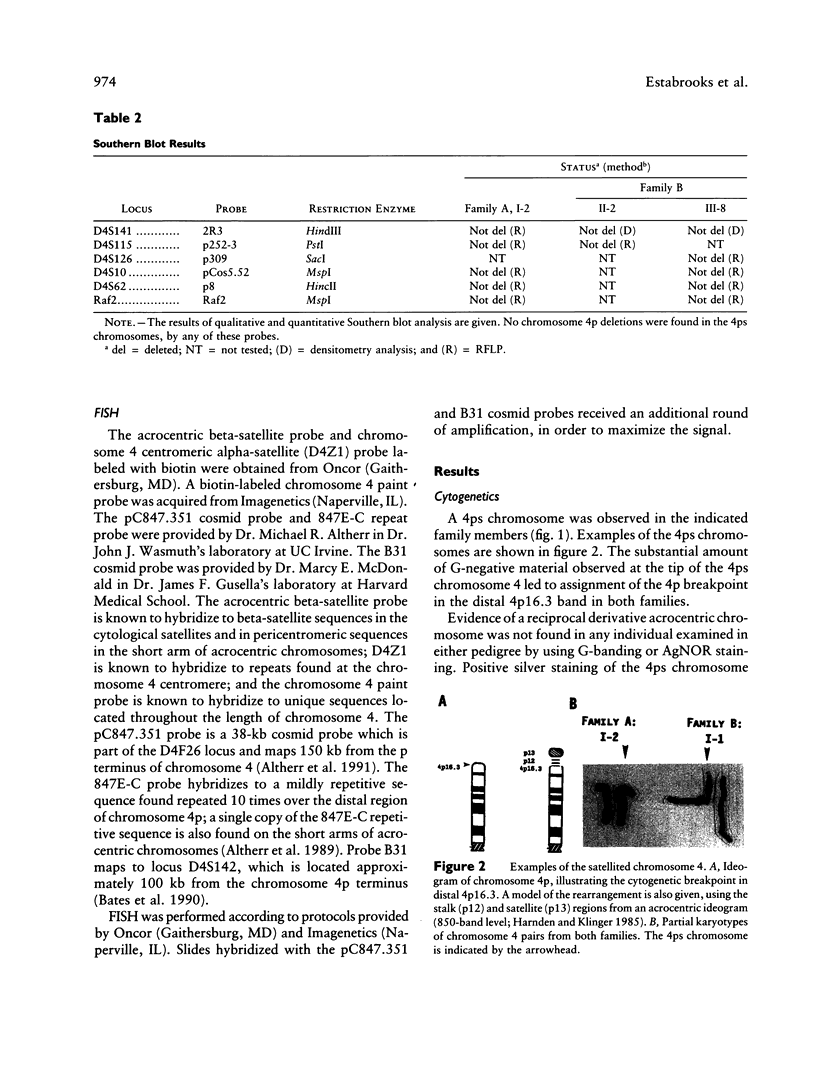
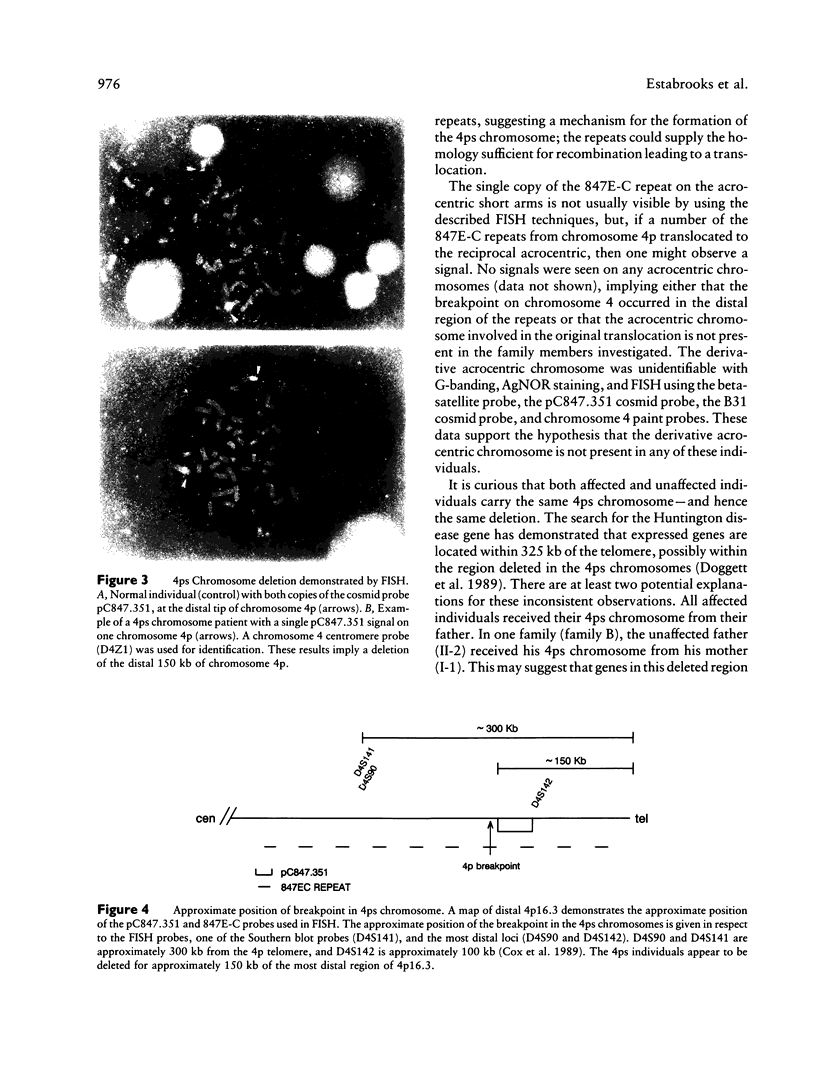
We report two families with a satellited chromosome 4 short arm (4ps). Satellites and stalks normally occur on the short arms of acrocentric chromosomes; however, the literature cites several reports of satellited nonacrocentric chromosomes, which presumably result from a translocation with an acrocentric chromosome. This is the first report of 4ps chromosomes. Our families are remarkable in that both unaffected and affected individuals carry the 4ps chromosome. The phenotypes observed in affected individuals, although dissimilar, were sufficient to encourage a search for a deletion of chromosome 4p. By Southern blot analysis and fluorescence in situ hybridization, a deletion of material mapping approximately 150 kb from chromosome 4pter was discovered. This deletion is notable because it does not result in the Wolf-Hirschhorn syndrome and can result in an apparently normal phenotype. We speculate that homology between subterminal repeat sequences on 4p and sequences on the acrocentric short arms may explain the origin of the rearrangement and that position effect may play a role in the expression of the abnormal phenotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altherr M. R., Bengtsson U., Elder F. F., Ledbetter D. H., Wasmuth J. J., McDonald M. E., Gusella J. F., Greenberg F. Molecular confirmation of Wolf-Hirschhorn syndrome with a subtle translocation of chromosome 4. Am J Hum Genet. 1991 Dec;49(6):1235–1242. [PMC free article] [PubMed] [Google Scholar]
- Altherr M. R., Smith B., MacDonald M. E., Hall L., Wasmuth J. J. Isolation of a novel mildly repetitive DNA sequence that is predominantly located at the terminus of the short arm of chromosome 4 near the Huntington disease gene. Genomics. 1989 Oct;5(3):581–588. doi: 10.1016/0888-7543(89)90026-8. [DOI] [PubMed] [Google Scholar]
- Bates G. P., MacDonald M. E., Baxendale S., Sedlacek Z., Youngman S., Romano D., Whaley W. L., Allitto B. A., Poustka A., Gusella J. F. A yeast artificial chromosome telomere clone spanning a possible location of the Huntington disease gene. Am J Hum Genet. 1990 Apr;46(4):762–775. [PMC free article] [PubMed] [Google Scholar]
- Cox D. R., Murray J. C., Buetow K. H. Report of the committee on the genetic constitution of chromosome 4. Cytogenet Cell Genet. 1989;51(1-4):121–136. doi: 10.1159/000132788. [DOI] [PubMed] [Google Scholar]
- Dev V. G., Byrne J., Bunch G. Partial translocation of NOR and its activity in a balanced carrier and in her cri-du-chat fetus. Hum Genet. 1979 Oct 2;51(3):277–280. doi: 10.1007/BF00283394. [DOI] [PubMed] [Google Scholar]
- Doggett N. A., Cheng J. F., Smith C. L., Cantor C. R. The Huntington disease locus is most likely within 325 kilobases of the chromosome 4p telomere. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10011–10014. doi: 10.1073/pnas.86.24.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro M., Lavia P. Activation of human ribosomal genes by 5-azacytidine. Exp Cell Res. 1983 May;145(2):452–457. doi: 10.1016/0014-4827(83)90024-1. [DOI] [PubMed] [Google Scholar]
- Ferraro M., Lavia P., Pelliccia F., de Capoa A. Clonal inheritance of rRNA gene activity: cytological evidence in human cells. Chromosoma. 1981;84(3):345–351. doi: 10.1007/BF00286024. [DOI] [PubMed] [Google Scholar]
- Haaf T., Hayman D. L., Schmid M. Quantitative determination of rDNA transcription units in vertebrate cells. Exp Cell Res. 1991 Mar;193(1):78–86. doi: 10.1016/0014-4827(91)90540-b. [DOI] [PubMed] [Google Scholar]
- Hansmann I., Wiedeking C., Grimm T., Gebauer J. Reciprocal or nonreciprocal human chromosome translocations? The identification of reciprocal translocations by silver staining. Hum Genet. 1977 Aug 31;38(1):1–5. doi: 10.1007/BF00295801. [DOI] [PubMed] [Google Scholar]
- Lucas M., Wallace I., Hirschhorn K. Recurrent abortions and chromosome abnormalities. J Obstet Gynaecol Br Commonw. 1972 Dec;79(12):1119–1127. doi: 10.1111/j.1471-0528.1972.tb11898.x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M., Basli A., Poulsen H. Nucleolus organizer regions in translocations involving acrocentric chromosomes. Cytogenet Cell Genet. 1980;26(1):14–21. doi: 10.1159/000131416. [DOI] [PubMed] [Google Scholar]
- Murray J. C., van Ommen G. B. Report of the committee on the genetic constitution of chromosome 4. Cytogenet Cell Genet. 1990;55(1-4):97–110. doi: 10.1159/000133000. [DOI] [PubMed] [Google Scholar]
- Parslow M., Chambers D., Drummond M., Hunter W. Two cases of trisomy 12p due to rcpt (12;21)(p11;p11) inherited through three generations. Hum Genet. 1979 Apr 5;47(3):253–260. doi: 10.1007/BF00321017. [DOI] [PubMed] [Google Scholar]
- Prieto F., Badía L., Beneyto M., Palau F. Nucleolus organizer regions (NORs) inserted in 6q15. Hum Genet. 1989 Feb;81(3):289–290. doi: 10.1007/BF00279007. [DOI] [PubMed] [Google Scholar]
- Reeves B. R., Casey G., Honeycombe J. R., Smith S. Correlation of differentiation state and silver staining of nucleolar organizers in the promyelocytic leukemia cell line HL-60. Cancer Genet Cytogenet. 1984 Oct;13(2):159–166. doi: 10.1016/0165-4608(84)90057-8. [DOI] [PubMed] [Google Scholar]
- Smetana K., Likovský Z. Nucleolar silver-stained granules in maturing erythroid and granulocytic cells. Cell Tissue Res. 1984;237(2):367–370. doi: 10.1007/BF00217159. [DOI] [PubMed] [Google Scholar]
- Stetten G., Sroka B., Schmidt M., Axelman J., Migeon B. R. Translocation of the nucleolus organizer region to the human X chromosome. Am J Hum Genet. 1986 Aug;39(2):245–252. [PMC free article] [PubMed] [Google Scholar]
- Takahashi R., Mihara K., Maeda S., Yamaguchi T., Chen H. L., Aoyama N., Murao S., Hatanaka M., Sugiyama T. Secondary activation of c-abl may be related to translocation to the nucleolar organizer region in an in vitro cultured rat leukemia cell line (K3D). Proc Natl Acad Sci U S A. 1986 Feb;83(4):1079–1083. doi: 10.1073/pnas.83.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley J. M., Gosden J., Hultén M. Familial reciprocal translocation t(9;13)(p11;p12) investigated by silver staining and in situ hybridisation. Hum Genet. 1981;59(4):422–428. doi: 10.1007/BF00295484. [DOI] [PubMed] [Google Scholar]
- Verellen-Dumoulin C., Freund M., De Meyer R., Laterre C., Frédéric J., Thompson M. W., Markovic V. D., Worton R. G. Expression of an X-linked muscular dystrophy in a female due to translocation involving Xp21 and non-random inactivation of the normal X chromosome. Hum Genet. 1984;67(1):115–119. doi: 10.1007/BF00270570. [DOI] [PubMed] [Google Scholar]
- Watt J. L., Couzin D. A., Lloyd D. J., Stephen G. S., McKay E. A familial insertion involving an active nucleolar organiser within chromosome 12. J Med Genet. 1984 Oct;21(5):379–384. doi: 10.1136/jmg.21.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. G., Towner J. W., Coffin G. S., Ebbin A. J., Siris E., Brager P. Genetic and clinical studies in 13 patients with the Wolf-Hirschhorn syndrome [del(4p)]. Hum Genet. 1981;59(4):297–307. doi: 10.1007/BF00295461. [DOI] [PubMed] [Google Scholar]
- van Tuinen P., Strong L. C., Pathak S. Reduced NOR association frequency in a 13/18 translocation chromosome. A family study. Hum Genet. 1983;65(1):82–84. doi: 10.1007/BF00285036. [DOI] [PubMed] [Google Scholar]