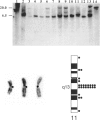
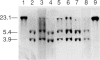
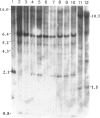
Abstract
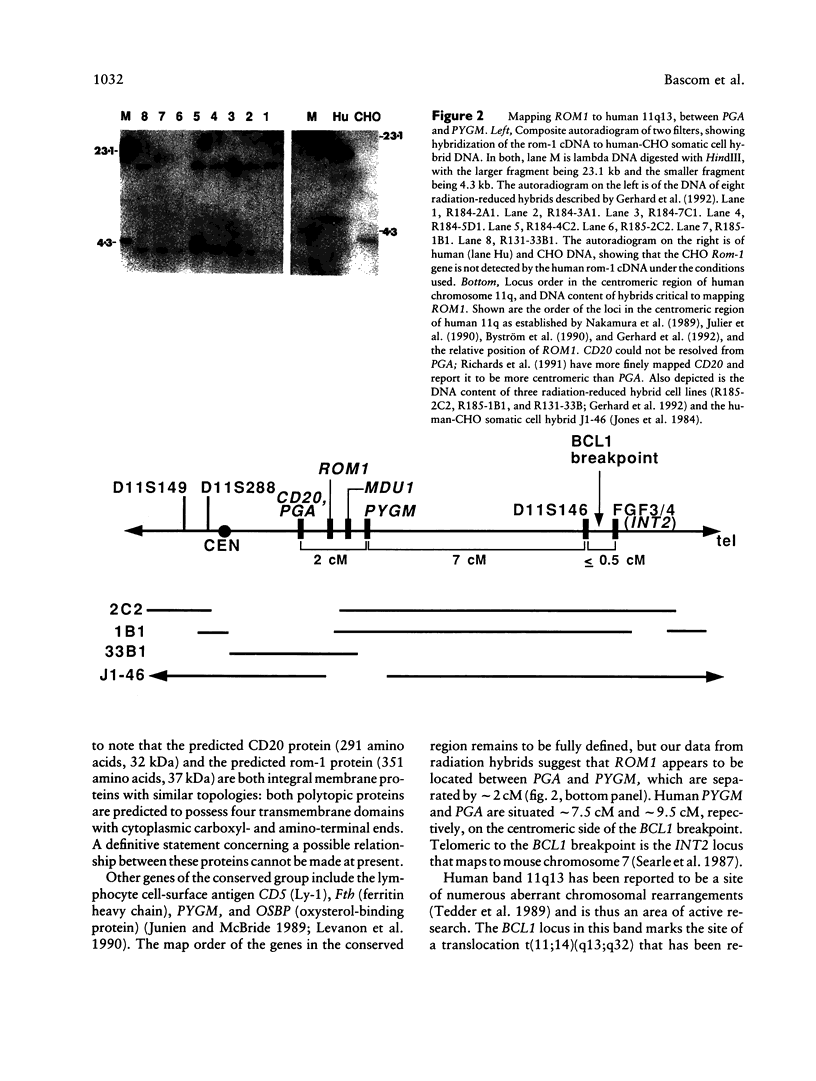
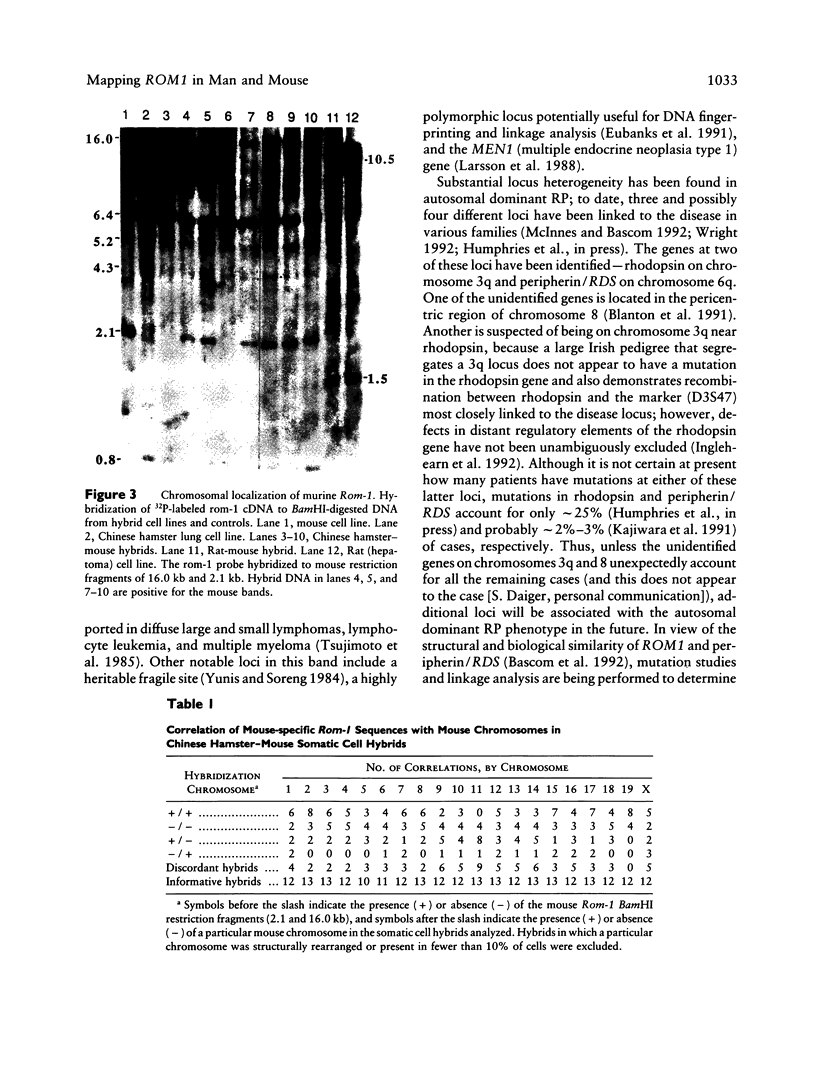
Rom-1 is a retinal integral membrane protein that, together with the product of the human retinal degeneration slow gene (RDS), defines a photoreceptor-specific protein family. The gene for rom-1 (HGM symbol: ROM1) has been assigned to human chromosome 11 and mouse chromosome 19 by Southern blot analysis of somatic cell hybrid DNAs. ROM1 was regionally sublocalized to human 11p13-11q13 by using three mouse-human somatic cell hybrids; in situ hybridization refined the sublocalization to human 11q13. Analysis of somatic cell hybrids suggested that the most likely localization of ROM1 is in the approximately 2-cM interval between human PGA (human pepsinogen A) and PYGM (muscle glycogen phosphorylase). ROM1 appears to be a new member of a conserved syntenic group whose members include such genes as CD5, CD20, and OSBP (oxysterol-binding protein), on human chromosome 11 and mouse chromosome 19. Localization of the ROM1 gene will permit the examination of its linkage to hereditary retinopathies in man and mouse.
Full text
PDF


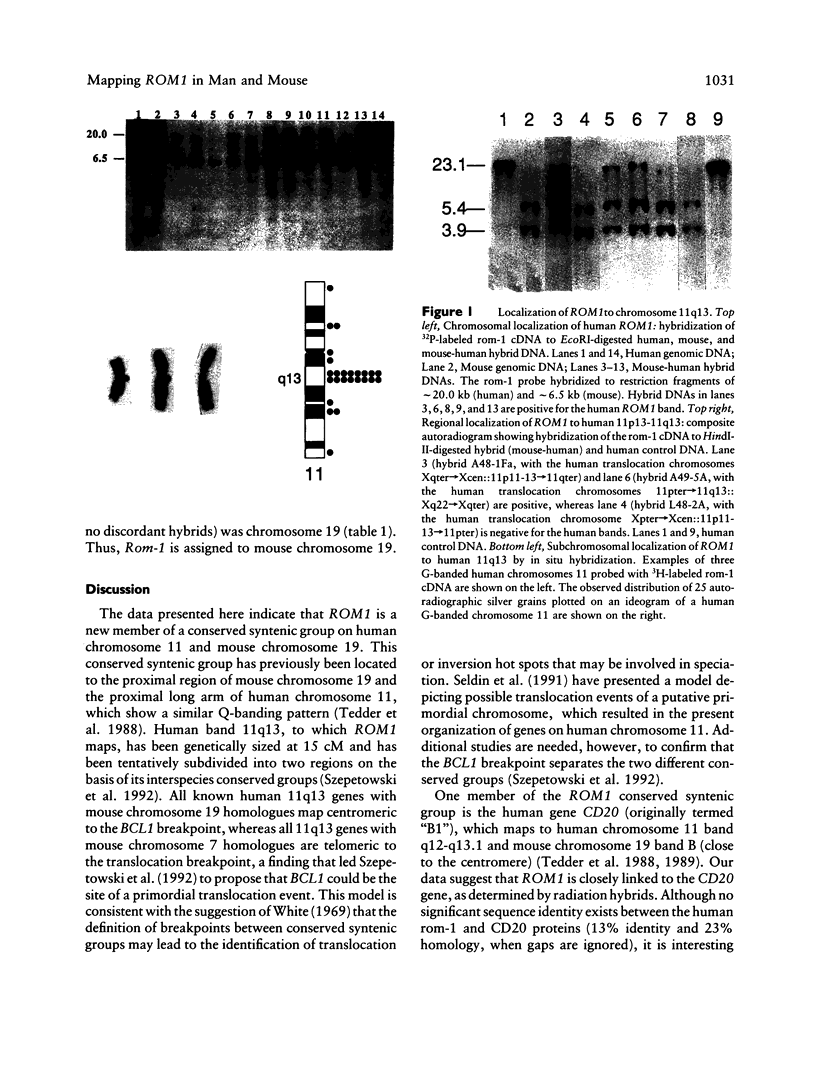


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascom R. A., Manara S., Collins L., Molday R. S., Kalnins V. I., McInnes R. R. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992 Jun;8(6):1171–1184. doi: 10.1016/0896-6273(92)90137-3. [DOI] [PubMed] [Google Scholar]
- Blanton S. H., Heckenlively J. R., Cottingham A. W., Friedman J., Sadler L. A., Wagner M., Friedman L. H., Daiger S. P. Linkage mapping of autosomal dominant retinitis pigmentosa (RP1) to the pericentric region of human chromosome 8. Genomics. 1991 Dec;11(4):857–869. doi: 10.1016/0888-7543(91)90008-3. [DOI] [PubMed] [Google Scholar]
- Byström C., Larsson C., Blomberg C., Sandelin K., Falkmer U., Skogseid B., Oberg K., Werner S., Nordenskjöld M. Localization of the MEN1 gene to a small region within chromosome 11q13 by deletion mapping in tumors. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1968–1972. doi: 10.1073/pnas.87.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro L. A., Emanuel B. S. An improved method for G-banding chromosomes after in situ hybridization. Cytogenet Cell Genet. 1984;38(4):308–309. doi: 10.1159/000132079. [DOI] [PubMed] [Google Scholar]
- Connell G. J., Molday R. S. Molecular cloning, primary structure, and orientation of the vertebrate photoreceptor cell protein peripherin in the rod outer segment disk membrane. Biochemistry. 1990 May 15;29(19):4691–4698. doi: 10.1021/bi00471a025. [DOI] [PubMed] [Google Scholar]
- Connell G., Bascom R., Molday L., Reid D., McInnes R. R., Molday R. S. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks J. H., Selleri L., Hart R., Rosette C., Evans G. A. Isolation, localization, and physical mapping of a highly polymorphic locus on human chromosome 11q13. Genomics. 1991 Nov;11(3):720–729. doi: 10.1016/0888-7543(91)90080-x. [DOI] [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gerhard D. S., Lawrence E., Wu J., Chua H., Ma N., Bland S., Jones C. Isolation of 1001 new markers from human chromosome 11, excluding the region of 11p13-p15.5, and their sublocalization by a new series of radiation-reduced somatic cell hybrids. Genomics. 1992 Aug;13(4):1133–1142. doi: 10.1016/0888-7543(92)90028-q. [DOI] [PubMed] [Google Scholar]
- Glaser T., Housman D., Lewis W. H., Gerhard D., Jones C. A fine-structure deletion map of human chromosome 11p: analysis of J1 series hybrids. Somat Cell Mol Genet. 1989 Nov;15(6):477–501. doi: 10.1007/BF01534910. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hawkins R. K., Jansen H. G., Sanyal S. Development and degeneration of retina in rds mutant mice: photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985 Dec;41(6):701–720. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Inglehearn C. F., Lester D. H., Bashir R., Atif U., Keen T. J., Sertedaki A., Lindsey J., Jay M., Bird A. C., Farrar G. J. Recombination between rhodopsin and locus D3S47 (C17) in rhodopsin retinitis pigmentosa families. Am J Hum Genet. 1992 Mar;50(3):590–597. [PMC free article] [PubMed] [Google Scholar]
- Jones C., Bill J., Larizza L., Pym B., Goodfellow P., Tunnacliffe A. Relationships between genes on human chromosome 11 encoding cell-surface antigens. Somat Cell Mol Genet. 1984 Jul;10(4):423–428. doi: 10.1007/BF01535638. [DOI] [PubMed] [Google Scholar]
- Jordan S. A., Farrar G. J., Kumar-Singh R., Kenna P., Humphries M. M., Allamand V., Sharp E. M., Humphries P. Autosomal dominant retinitis pigmentosa (adRP; RP6): cosegregation of RP6 and the peripherin-RDS locus in a late-onset family of Irish origin. Am J Hum Genet. 1992 Mar;50(3):634–639. [PMC free article] [PubMed] [Google Scholar]
- Julier C., Nakamura Y., Lathrop M., O'Connell P., Leppert M., Litt M., Mohandas T., Lalouel J. M., White R. A detailed genetic map of the long arm of chromosome 11. Genomics. 1990 Jul;7(3):335–345. doi: 10.1016/0888-7543(90)90167-s. [DOI] [PubMed] [Google Scholar]
- Junien C., McBride O. W. Report of the committee on the genetic constitution of chromosome 11. Cytogenet Cell Genet. 1989;51(1-4):226–258. doi: 10.1159/000132793. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Hahn L. B., Mukai S., Travis G. H., Berson E. L., Dryja T. P. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Lafrenière R. G., Brown C. J., Powers V. E., Carrel L., Davies K. E., Barker D. F., Willard H. F. Physical mapping of 60 DNA markers in the p21.1----q21.3 region of the human X chromosome. Genomics. 1991 Oct;11(2):352–363. doi: 10.1016/0888-7543(91)90143-3. [DOI] [PubMed] [Google Scholar]
- Larsson C., Skogseid B., Oberg K., Nakamura Y., Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988 Mar 3;332(6159):85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Levanon D., Hsieh C. L., Francke U., Dawson P. A., Ridgway N. D., Brown M. S., Goldstein J. L. cDNA cloning of human oxysterol-binding protein and localization of the gene to human chromosome 11 and mouse chromosome 19. Genomics. 1990 May;7(1):65–74. doi: 10.1016/0888-7543(90)90519-z. [DOI] [PubMed] [Google Scholar]
- McInnes R. R., Bascom R. A. Retinal genetics: a nullifying effect for rhodopsin. Nat Genet. 1992 Jun;1(3):155–157. doi: 10.1038/ng0692-155. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Larsson C., Julier C., Byström C., Skogseid B., Wells S., Oberg K., Carlson M., Taggart T., O'Connell P. Localization of the genetic defect in multiple endocrine neoplasia type 1 within a small region of chromosome 11. Am J Hum Genet. 1989 May;44(5):751–755. [PMC free article] [PubMed] [Google Scholar]
- Richard C. W., 3rd, Withers D. A., Meeker T. C., Maurer S., Evans G. A., Myers R. M., Cox D. R. A radiation hybrid map of the proximal long arm of human chromosome 11 containing the multiple endocrine neoplasia type 1 (MEN-1) and bcl-1 disease loci. Am J Hum Genet. 1991 Dec;49(6):1189–1196. [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., De Ruiter A., Hawkins R. K. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neurol. 1980 Nov 1;194(1):193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Searle A. G., Peters J., Lyon M. F., Evans E. P., Edwards J. H., Buckle V. J. Chromosome maps of man and mouse, III. Genomics. 1987 Sep;1(1):3–18. doi: 10.1016/0888-7543(87)90099-1. [DOI] [PubMed] [Google Scholar]
- Seldin M. F., Saunders A. M., Rochelle J. M., Howard T. A. A proximal mouse chromosome 9 linkage map that further defines linkage groups homologous with segments of human chromosomes 11, 15, and 19. Genomics. 1991 Apr;9(4):678–685. doi: 10.1016/0888-7543(91)90361-h. [DOI] [PubMed] [Google Scholar]
- Szepetowski P., Simon M. P., Grosgeorge J., Huebner K., Bastard C., Evans G. A., Tsujimoto Y., Birnbaum D., Theillet C., Gaudray P. Localization of 11q13 loci with respect to regional chromosomal breakpoints. Genomics. 1992 Apr;12(4):738–744. doi: 10.1016/0888-7543(92)90303-a. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Disteche C. M., Louie E., Adler D. A., Croce C. M., Schlossman S. F., Saito H. The gene that encodes the human CD20 (B1) differentiation antigen is located on chromosome 11 near the t(11;14)(q13;q32) translocation site. J Immunol. 1989 Apr 1;142(7):2555–2559. [PubMed] [Google Scholar]
- Tedder T. F., Klejman G., Disteche C. M., Adler D. A., Schlossman S. F., Saito H. Cloning of a complementary DNA encoding a new mouse B lymphocyte differentiation antigen, homologous to the human B1 (CD20) antigen, and localization of the gene to chromosome 19. J Immunol. 1988 Dec 15;141(12):4388–4394. [PubMed] [Google Scholar]
- Travis G. H., Brennan M. B., Danielson P. E., Kozak C. A., Sutcliffe J. G. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds). Nature. 1989 Mar 2;338(6210):70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- Travis G. H., Christerson L., Danielson P. E., Klisak I., Sparkes R. S., Hahn L. B., Dryja T. P., Sutcliffe J. G. The human retinal degeneration slow (RDS) gene: chromosome assignment and structure of the mRNA. Genomics. 1991 Jul;10(3):733–739. doi: 10.1016/0888-7543(91)90457-p. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Wright A. F. New insights into genetic eye disease. Trends Genet. 1992 Mar;8(3):85–91. doi: 10.1016/0168-9525(92)90195-a. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., DeGennaro L. J., Francke U. Genes for synapsin I, a neuronal phosphoprotein, map to conserved regions of human and murine X chromosomes. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8679–8683. doi: 10.1073/pnas.83.22.8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Soreng A. L. Constitutive fragile sites and cancer. Science. 1984 Dec 7;226(4679):1199–1204. doi: 10.1126/science.6239375. [DOI] [PubMed] [Google Scholar]
- van Nie R., Iványi D., Démant P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens. 1978 Aug;12(2):106–108. doi: 10.1111/j.1399-0039.1978.tb01305.x. [DOI] [PubMed] [Google Scholar]