Abstract
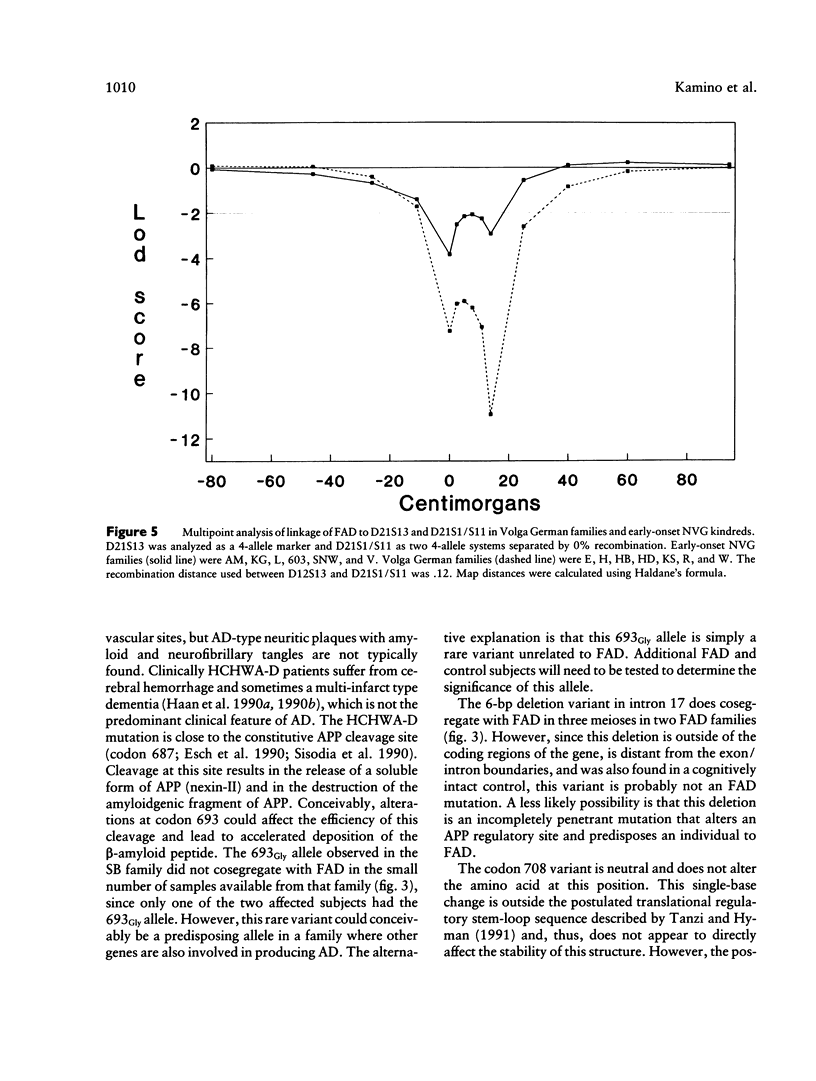
A large number of familial Alzheimer disease (FAD) kindreds were examined to determine whether mutations in the amyloid precursor protein (APP) gene could be responsible for the disease. Previous studies have identified three mutations at APP codon 717 which are pathogenic for Alzheimer disease (AD). Samples from affected subjects were examined for mutations in exons 16 and 17 of the APP gene. A combination of direct sequencing and single-strand conformational polymorphism analysis was used. Sporadic AD and normal controls were also examined by the same methods. Five sequence variants were identified. One variant at APP codon 693 resulted in a Glu→Gly change. This is the same codon as the hereditary cerebral hemorrhage with amyloidosis–Dutch type Glu→Gln mutation. Another single-base change at APP codon 708 did not alter the amino acid encoded at this site. Two point mutations and a 6-bp deletion were identified in the intronic sequences surrounding exon 17. None of the variants could be unambiguously determined to be responsible for FAD. The larger families were also analyzed by testing for linkage of FAD to a highly polymorphic short tandem repeat marker (D21S210) that is tightly linked to APP. Highly negative LOD scores were obtained for the family groups tested, and linkage was formally excluded beyond θ = .10 for the Volga German kindreds, θ = .20 for early-onset non-Volga Germans, and θ = .10 for late-onset families. LOD scores for linkage of FAD to markers centromeric to APP (D21S1/S11, D21S13, and D21S215) were also negative in the three family groups. These studies show that APP mutations account for AD in only a small fraction of FAD kindreds.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird T. D., Lampe T. H., Nemens E. J., Miner G. W., Sumi S. M., Schellenberg G. D. Familial Alzheimer's disease in American descendants of the Volga Germans: probable genetic founder effect. Ann Neurol. 1988 Jan;23(1):25–31. doi: 10.1002/ana.410230106. [DOI] [PubMed] [Google Scholar]
- Bird T. D., Sumi S. M., Nemens E. J., Nochlin D., Schellenberg G., Lampe T. H., Sadovnick A., Chui H., Miner G. W., Tinklenberg J. Phenotypic heterogeneity in familial Alzheimer's disease: a study of 24 kindreds. Ann Neurol. 1989 Jan;25(1):12–25. doi: 10.1002/ana.410250104. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Crawford F., Hamandi K., Mullan M., Goate A., Hardy J., Backhovens H., Martin J. J., Broeckhoven C. V. Screening for the beta-amyloid precursor protein mutation (APP717: Val----Ile) in extended pedigrees with early onset Alzheimer's disease. Neurosci Lett. 1991 Aug 5;129(1):134–135. doi: 10.1016/0304-3940(91)90738-f. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991 Oct 31;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Cook R. H., Ward B. E., Austin J. H. Studies in aging of the brain: IV. Familial Alzheimer disease: Relation to transmissible dementia, aneuploidy, and microtubular defects. Neurology. 1979 Oct;29(10):1402–1412. doi: 10.1212/wnl.29.10.1402. [DOI] [PubMed] [Google Scholar]
- Crawford F., Hardy J., Mullan M., Goate A., Hughes D., Fidani L., Roques P., Rossor M., Chartier-Harlin M. C. Sequencing of exons 16 and 17 of the beta-amyloid precursor protein gene in 14 families with early onset Alzheimer's disease fails to reveal mutations in the beta-amyloid sequence. Neurosci Lett. 1991 Nov 25;133(1):1–2. doi: 10.1016/0304-3940(91)90042-r. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., White B. J., Weitkamp L. R., Keats B. J., Morrow C. H., Gajdusek D. C. Familial Alzheimer's disease in two kindreds of the same geographic and ethnic origin. A clinical and genetic study. J Neurol Sci. 1981 Jan;49(1):79–89. doi: 10.1016/0022-510x(81)90190-8. [DOI] [PubMed] [Google Scholar]
- Guo Z., Sharma V., Patterson D., Litt M. TG repeat polymorphism at the D21S167 locus. Nucleic Acids Res. 1990 Aug 25;18(16):4967–4967. doi: 10.1093/nar/18.16.4967-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez Valencia L., Alonso E., Figueroa H. H., Escobar A. Enfermedad de Alzheimer. Presentación de 7 casos en 3 familias. Rev Invest Clin. 1986 Jul-Sep;38(3):261–267. [PubMed] [Google Scholar]
- Haan J., Lanser J. B., Zijderveld I., van der Does I. G., Roos R. A. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type. Arch Neurol. 1990 Sep;47(9):965–967. doi: 10.1001/archneur.1990.00530090035010. [DOI] [PubMed] [Google Scholar]
- Heston L. L., Orr H. T., Rich S. S., White J. A. Linkage of an Alzheimer disease susceptibility locus to markers on human chromosome 21. Am J Med Genet. 1991 Sep 15;40(4):449–453. doi: 10.1002/ajmg.1320400415. [DOI] [PubMed] [Google Scholar]
- Hodge S. E., Anderson C. E., Neiswanger K., Sparkes R. S., Rimoin D. L. The search for heterogeneity in insulin-dependent diabetes mellitus (IDDM): linkage studies, two-locus models, and genetic heterogeneity. Am J Hum Genet. 1983 Nov;35(6):1139–1155. [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire H. G., Salbaum J. M., Multhaup G., Kang J., Bayney R. M., Unterbeck A., Beyreuther K., Müller-Hill B. The PreA4(695) precursor protein of Alzheimer's disease A4 amyloid is encoded by 16 exons. Nucleic Acids Res. 1989 Jan 25;17(2):517–522. doi: 10.1093/nar/17.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E., Carman M. D., Fernandez-Madrid I. J., Power M. D., Lieberburg I., van Duinen S. G., Bots G. T., Luyendijk W., Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- MORTON N. E. The detection and estimation of linkage between the genes for elliptocytosis and the Rh blood type. Am J Hum Genet. 1956 Jun;8(2):80–96. [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Murrell J., Farlow M., Ghetti B., Benson M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Naruse S., Igarashi S., Kobayashi H., Aoki K., Inuzuka T., Kaneko K., Shimizu T., Iihara K., Kojima T., Miyatake T. Mis-sense mutation Val----Ile in exon 17 of amyloid precursor protein gene in Japanese familial Alzheimer's disease. Lancet. 1991 Apr 20;337(8747):978–979. doi: 10.1016/0140-6736(91)91612-x. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance M. A., Bebout J. L., Gaskell P. C., Jr, Yamaoka L. H., Hung W. Y., Alberts M. J., Walker A. P., Bartlett R. J., Haynes C. A., Welsh K. A. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991 Jun;48(6):1034–1050. [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Anderson L., O'dahl S., Wisjman E. M., Sadovnick A. D., Ball M. J., Larson E. B., Kukull W. A., Martin G. M., Roses A. D. APP717, APP693, and PRIP gene mutations are rare in Alzheimer disease. Am J Hum Genet. 1991 Sep;49(3):511–517. [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Moore D. K., Boehnke M., Bryant E. M., Lampe T. H., Nochlin D., Sumi S. M., Deeb S. S. Absence of linkage of chromosome 21q21 markers to familial Alzheimer's disease. Science. 1988 Sep 16;241(4872):1507–1510. doi: 10.1126/science.3420406. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Boehnke M., Wijsman E. M., Moore D. K., Martin G. M., Bird T. D. Genetic association and linkage analysis of the apolipoprotein CII locus and familial Alzheimer's disease. Ann Neurol. 1992 Feb;31(2):223–227. doi: 10.1002/ana.410310214. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Pericak-Vance M. A., Wijsman E. M., Moore D. K., Gaskell P. C., Jr, Yamaoka L. A., Bebout J. L., Anderson L., Welsh K. A., Clark C. M. Linkage analysis of familial Alzheimer disease, using chromosome 21 markers. Am J Hum Genet. 1991 Mar;48(3):563–583. [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Litt M. Tetranucleotide repeat polymorphism at the D21S11 locus. Hum Mol Genet. 1992 Apr;1(1):67–67. doi: 10.1093/hmg/1.1.67-a. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Haines J. L., Farrer L. A., Polinsky R., Van Broeckhoven C., Goate A., McLachlan D. R., Orr H., Bruni A. C., Sorbi S. Genetic linkage studies suggest that Alzheimer's disease is not a single homogeneous disorder. Nature. 1990 Sep 13;347(6289):194–197. doi: 10.1038/347194a0. [DOI] [PubMed] [Google Scholar]
- Stinissen P., Van Broeckhoven C. A new (CA)n repeat polymorphism at the D21S13E locus. Nucleic Acids Res. 1991 Sep 25;19(18):5089–5089. doi: 10.1093/nar/19.18.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., George-Hyslop P. S., Gusella J. F. Molecular genetics of Alzheimer disease amyloid. J Biol Chem. 1991 Nov 5;266(31):20579–20582. [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Hyman B. T. Alzheimer's mutation. Nature. 1991 Apr 18;350(6319):564–564. doi: 10.1038/350564a0. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., St George-Hyslop P. H., Haines J. L., Polinsky R. J., Nee L., Foncin J. F., Neve R. L., McClatchey A. I., Conneally P. M., Gusella J. F. The genetic defect in familial Alzheimer's disease is not tightly linked to the amyloid beta-protein gene. Nature. 1987 Sep 10;329(6135):156–157. doi: 10.1038/329156a0. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Watkins P. C., Stewart G. D., Wexler N. S., Gusella J. F., Haines J. L. A genetic linkage map of human chromosome 21: analysis of recombination as a function of sex and age. Am J Hum Genet. 1992 Mar;50(3):551–558. [PMC free article] [PubMed] [Google Scholar]
- Van Broeckhoven C., Genthe A. M., Vandenberghe A., Horsthemke B., Backhovens H., Raeymaekers P., Van Hul W., Wehnert A., Gheuens J., Cras P. Failure of familial Alzheimer's disease to segregate with the A4-amyloid gene in several European families. Nature. 1987 Sep 10;329(6135):153–155. doi: 10.1038/329153a0. [DOI] [PubMed] [Google Scholar]
- Warren A. C., Petersen M. B., Van Hul W., McInnis M. G., Van Broeckhoven C., Cox T. K., Chakravarti A., Antonarakis S. E. D21S215 is a (GT)n polymorphic marker close to centromeric alphoid sequences on chromosome 21. Genomics. 1992 Aug;13(4):1365–1367. doi: 10.1016/0888-7543(92)90072-z. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Miki T., Katsuya T., Ogihara T., Sakaki Y. The 717Val----Ile substitution in amyloid precursor protein is associated with familial Alzheimer's disease regardless of ethnic groups. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1141–1146. doi: 10.1016/0006-291x(91)91011-z. [DOI] [PubMed] [Google Scholar]
- de Sauvage F., Octave J. N. A novel mRNA of the A4 amyloid precursor gene coding for a possibly secreted protein. Science. 1989 Aug 11;245(4918):651–653. doi: 10.1126/science.2569763. [DOI] [PubMed] [Google Scholar]
- van Duijn C. M., Hendriks L., Cruts M., Hardy J. A., Hofman A., Van Broeckhoven C. Amyloid precursor protein gene mutation in early-onset Alzheimer's disease. Lancet. 1991 Apr 20;337(8747):978–978. doi: 10.1016/0140-6736(91)91611-w. [DOI] [PubMed] [Google Scholar]




