Abstract
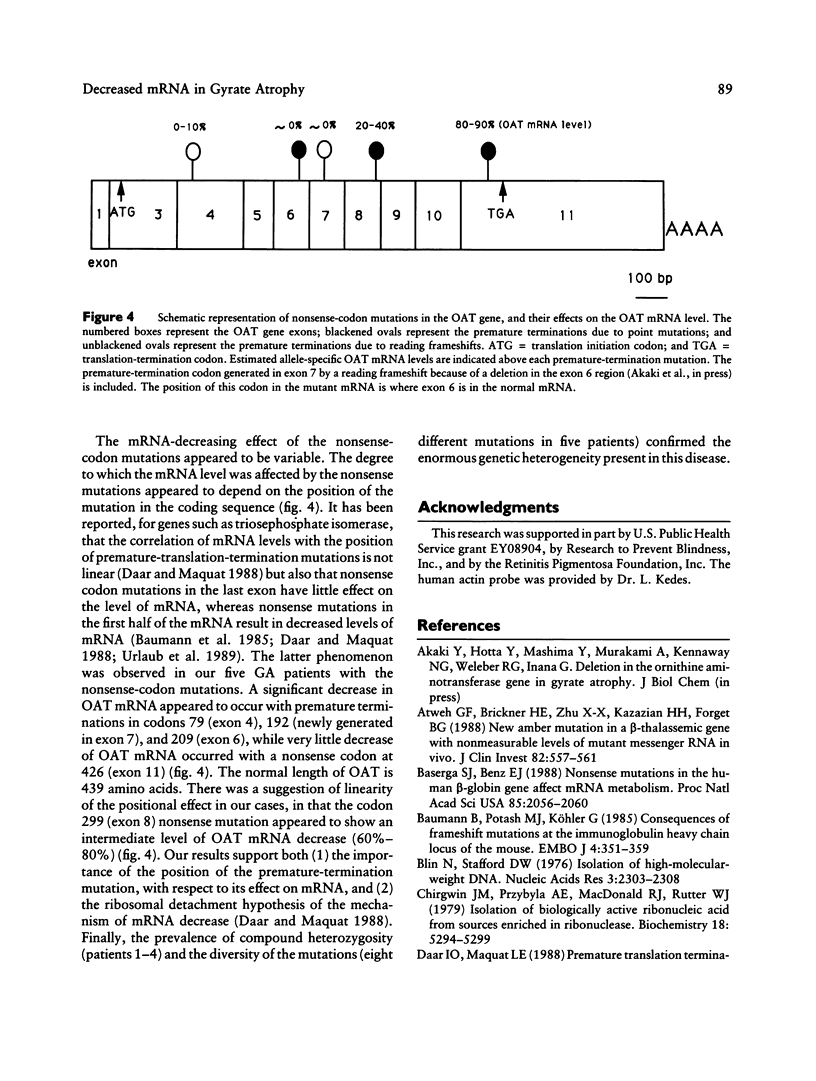
A generalized deficiency of the mitochondrial matrix enzyme ornithine aminotransferase (OAT) is the inborn error in gyrate atrophy (GA), an autosomal recessive degenerative disease of the retina and choroid of the eye. Mutations in the OAT gene show a high degree of molecular heterogeneity in GA, reflecting the genetic heterogeneity in this disease. Using the combined techniques of PCR, denaturing gradient gel electrophoresis, and direct sequencing, we have identified three nonsense-codon mutations and one nonsense codon-generating mutation of the OAT gene in GA pedigrees. Three of them are single-base substitutions, and one is a 2-bp deletion resulting in a reading frameshift. A nonsense codon created at position 79 (TGA) by a frameshift and nonsense mutations at codons 209 (TAT----TAA) and 299 (TAC----TAG) result in abnormally low levels of OAT mRNA in the patient's skin fibroblasts. A nonsense mutation at codon 426 (CGA----TGA) in the last exon, however, has little effect on the mRNA level. Thus, the mRNA level can be reduced by nonsense-codon mutations, but the position of the mutation may be important, with earlier premature-translation termination having a greater effect than a later mutation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atweh G. F., Brickner H. E., Zhu X. X., Kazazian H. H., Jr, Forget B. G. New amber mutation in a beta-thalassemic gene with nonmeasurable levels of mutant messenger RNA in vivo. J Clin Invest. 1988 Aug;82(2):557–561. doi: 10.1172/JCI113632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo S. S., Stalenhoef A. F., Marr K., Gregg R. E., Ross R. S., Brewer H. B., Jr A deletion mutation in the ApoC-II gene (ApoC-II Nijmegen) of a patient with a deficiency of apolipoprotein C-II. J Biol Chem. 1988 Dec 5;263(34):17913–17916. [PubMed] [Google Scholar]
- Hayasaka S., Saito T., Nakajima H., Takaku Y., Shiono T., Mizuno K., Ohmura K., Tada K. Gyrate atrophy with hyperornithinaemia: different types of responsiveness to vitamin B6. Br J Ophthalmol. 1981 Jul;65(7):478–483. doi: 10.1136/bjo.65.7.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs D. R., Goodbourn S. E., Lamb J., Clegg J. B., Weatherall D. J., Proudfoot N. J. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983 Nov 24;306(5941):398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Russell D. W., Davignon J., Goldstein J. L. Deletion in the gene for the low-density-lipoprotein receptor in a majority of French Canadians with familial hypercholesterolemia. N Engl J Med. 1987 Sep 17;317(12):734–737. doi: 10.1056/NEJM198709173171204. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Kennaway N. G., Weleber R. G., Inana G. Inheritance of ornithine aminotransferase gene, mRNA, and enzyme defect in a family with gyrate atrophy of the choroid and retina. Am J Hum Genet. 1989 Mar;44(3):353–357. [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Ley T. J., Anagnou N. P., Baur A. W., Nienhuis A. W. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood. 1984 Jul;64(1):23–32. [PubMed] [Google Scholar]
- Humphries R. K., Ley T. J., Anagnou N. P., Baur A. W., Nienhuis A. W. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood. 1984 Jul;64(1):23–32. [PubMed] [Google Scholar]
- Inana G., Chambers C., Hotta Y., Inouye L., Filpula D., Pulford S., Shiono T. Point mutation affecting processing of the ornithine aminotransferase precursor protein in gyrate atrophy. J Biol Chem. 1989 Oct 15;264(29):17432–17436. [PubMed] [Google Scholar]
- Inana G., Hotta Y., Zintz C., Takki K., Weleber R. G., Kennaway N. G., Nakayasu K., Nakajima A., Shiono T. Expression defect of ornithine aminotransferase gene in gyrate atrophy. Invest Ophthalmol Vis Sci. 1988 Jul;29(7):1001–1005. [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. L., Ramesh V., McClatchey A. I., Menkes J. H., Tobin A. J. Detection of point mutations associated with genetic diseases by an exon scanning technique. Genomics. 1990 Dec;8(4):656–663. doi: 10.1016/0888-7543(90)90252-p. [DOI] [PubMed] [Google Scholar]
- Kennaway N. G., Stankova L., Wirtz M. K., Weleber R. G. Gyrate atrophy of the choroid and retina: characterization of mutant ornithine aminotransferase and mechanism of response to vitamin B6. Am J Hum Genet. 1989 Mar;44(3):344–352. [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- Lim S., Mullins J. J., Chen C. M., Gross K. W., Maquat L. E. Novel metabolism of several beta zero-thalassemic beta-globin mRNAs in the erythroid tissues of transgenic mice. EMBO J. 1989 Sep;8(9):2613–2619. doi: 10.1002/j.1460-2075.1989.tb08401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I., Kaufman D. L., Berson E. L., Tobin A. J., Shih V. E., Gusella J. F., Ramesh V. Splicing defect at the ornithine aminotransferase (OAT) locus in gyrate atrophy. Am J Hum Genet. 1990 Nov;47(5):790–794. [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Brody L. C., Looney J., Steel G., Suchanek M., Dowling C., Der Kaloustian V., Kaiser-Kupfer M., Valle D. An initiator codon mutation in ornithine-delta-aminotransferase causing gyrate atrophy of the choroid and retina. J Clin Invest. 1988 Feb;81(2):630–633. doi: 10.1172/JCI113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Brody L. C., Sipila I., Looney J. E., Wong C., Engelhardt J. F., Patel A. S., Steel G., Obie C., Kaiser-Kupfer M. At least two mutant alleles of ornithine delta-aminotransferase cause gyrate atrophy of the choroid and retina in Finns. Proc Natl Acad Sci U S A. 1989 Jan;86(1):197–201. doi: 10.1073/pnas.86.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Newton C. R., Kalsheker N., Graham A., Powell S., Gammack A., Riley J., Markham A. F. Diagnosis of alpha 1-antitrypsin deficiency by enzymatic amplification of human genomic DNA and direct sequencing of polymerase chain reaction products. Nucleic Acids Res. 1988 Sep 12;16(17):8233–8243. doi: 10.1093/nar/16.17.8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh V., McClatchey A. I., Ramesh N., Benoit L. A., Berson E. L., Shih V. E., Gusella J. F. Molecular basis of ornithine aminotransferase deficiency in B-6-responsive and -nonresponsive forms of gyrate atrophy. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3777–3780. doi: 10.1073/pnas.85.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traystman M. D., Higuchi M., Kasper C. K., Antonarakis S. E., Kazazian H. H., Jr Use of denaturing gradient gel electrophoresis to detect point mutations in the factor VIII gene. Genomics. 1990 Feb;6(2):293–301. doi: 10.1016/0888-7543(90)90569-g. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle D., Kaiser-Kupfer M. I., Del Valle L. A. Gyrate atrophy of the choroid and retina: deficiency of ornithine aminotransferase in transformed lymphocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5159–5161. doi: 10.1073/pnas.74.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weleber R. G., Tobler W. R. Computerized quantitative analysis of kinetic visual fields. Am J Ophthalmol. 1986 Apr 15;101(4):461–468. doi: 10.1016/0002-9394(86)90648-3. [DOI] [PubMed] [Google Scholar]
- Weleber R. G., Wirtz M. K., Kennaway N. G. Gyrate atrophy of the choroid and retina: clinical and biochemical heterogeneity and response to vitamin B6. Birth Defects Orig Artic Ser. 1982;18(6):219–230. [PubMed] [Google Scholar]