Abstract
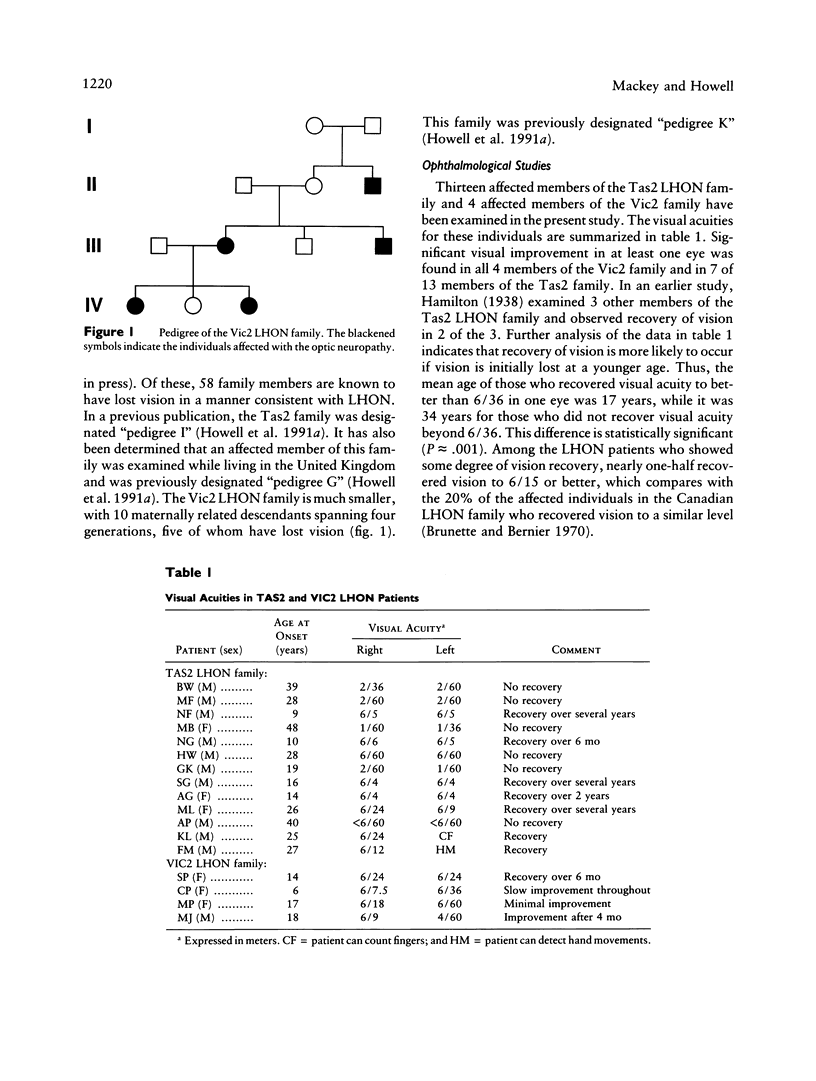
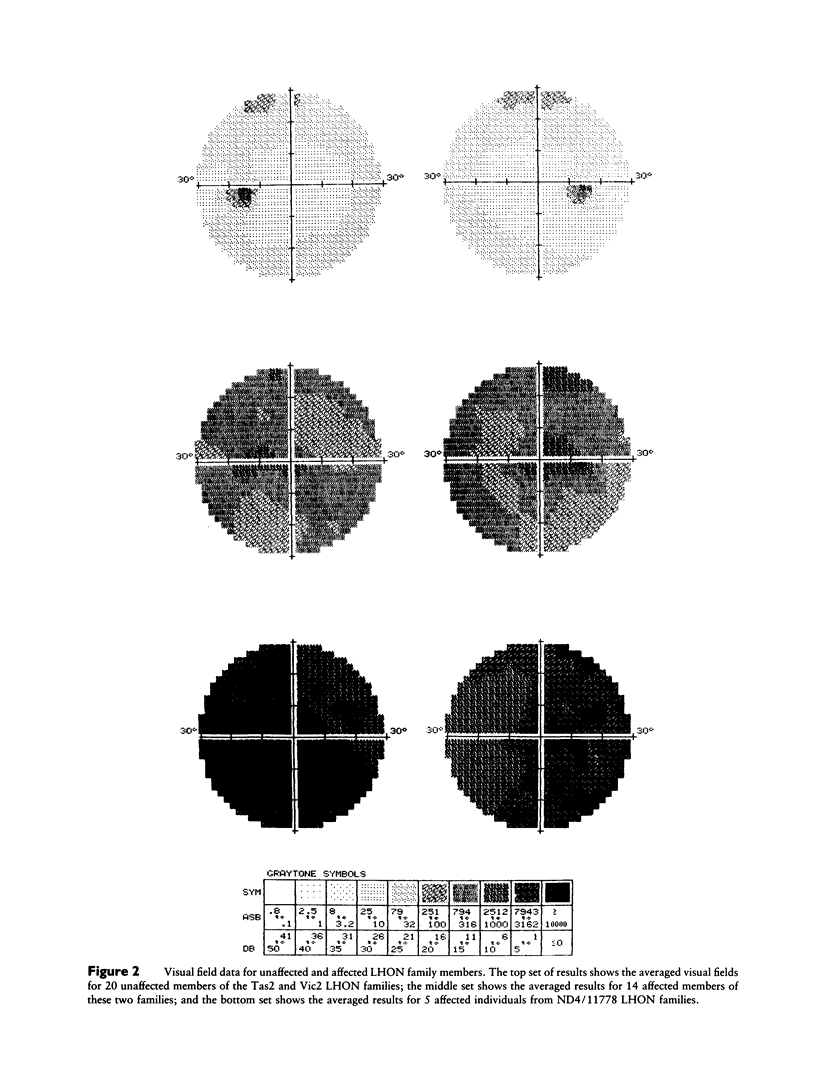
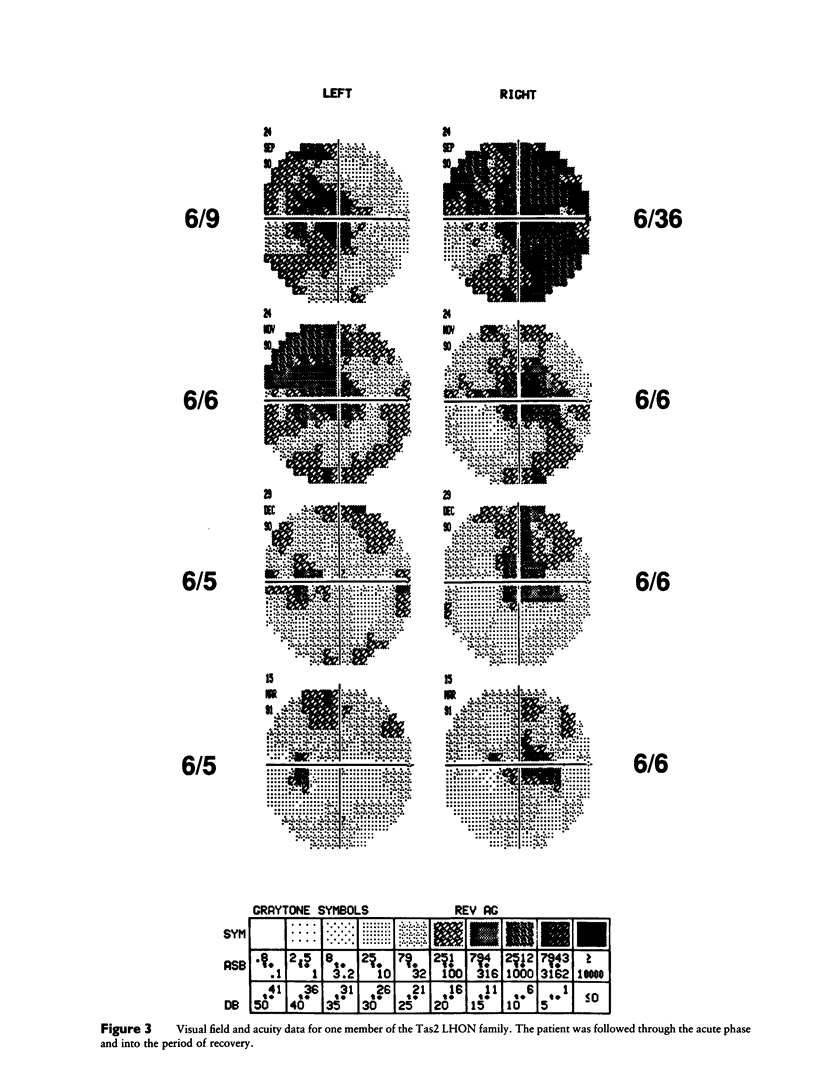
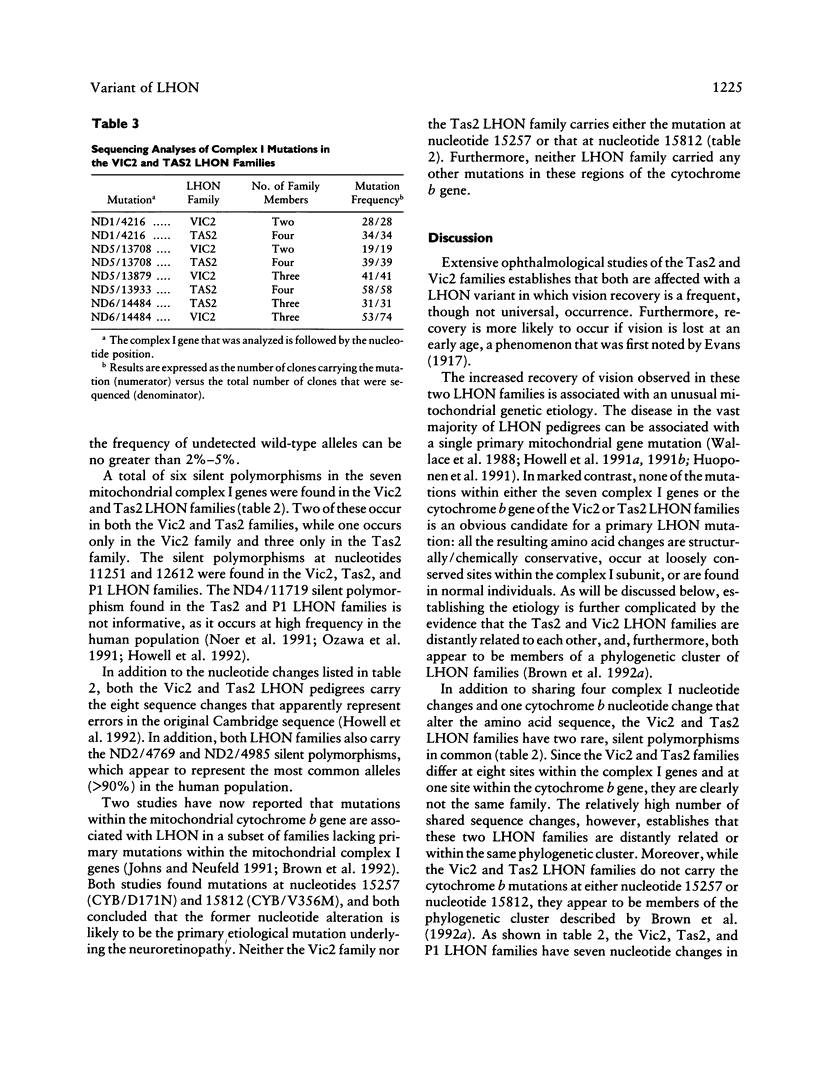
The Tas2 and Vic2 Australian families are affected with a variant of Leber hereditary optic neuropathy (LHON). The risk of developing the optic neuropathy shows strict maternal inheritance, and the ophthalmological changes in affected family members are characteristic of LHON. However, in contrast to the common form of the disease, members of these two families show a high frequency of vision recovery. To ascertain the mitochondrial genetic etiology of the LHON in these families, both (a) the the nucleotide sequences of the seven mitochondrial genes encoding subunits of respiratory-chain complex I and (b) the mitochondrial cytochrome b gene were determined for representatives of both families. Neither family carries any of the previously identified primary mitochondrial LHON mutations: ND4/11778, ND1/3460, or ND1/4160. Instead, both LHON families carry multiple nucleotide changes in the mitochondrial complex I genes, which produce conservative amino acid changes. From the available sequence data, it is inferred that the Vic2 and Tas2 LHON families are phylogenetically related to each other and to a cluster of LHON families in which mutations in the mitochondrial cytochrome b gene have been hypothesized to play a primary etiological role. However, sequencing analysis establishes that the Vic2 and Tas2 LHON families do not carry these cytochrome b mutations. There are two hypotheses to account for the unusual mitochondrial genetic etiology of the LHON in the Tas2 and Vic2 LHON families. One possibility is that there is a primary LHON mutation within the mitochondrial genome but that it is at a site that was not included in the sequencing analyses. Alternatively, the disease in these families may result from the cumulative effects of multiple secondary LHON mutations that have less severe phenotypic consequences.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Brown M. D., Voljavec A. S., Lott M. T., Torroni A., Yang C. C., Wallace D. C. Mitochondrial DNA complex I and III mutations associated with Leber's hereditary optic neuropathy. Genetics. 1992 Jan;130(1):163–173. doi: 10.1093/genetics/130.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. D., Yang C. C., Trounce I., Torroni A., Lott M. T., Wallace D. C. A mitochondrial DNA variant, identified in Leber hereditary optic neuropathy patients, which extends the amino acid sequence of cytochrome c oxidase subunit I. Am J Hum Genet. 1992 Aug;51(2):378–385. [PMC free article] [PubMed] [Google Scholar]
- Brunette J. R., Bernier R. G. Diagnostic et pronostic de la maladie de Leber: incidence de la récupération totale spontanée. Union Med Can. 1970 Apr;99(4):643–652. [PubMed] [Google Scholar]
- Holt I. J., Miller D. H., Harding A. E. Genetic heterogeneity and mitochondrial DNA heteroplasmy in Leber's hereditary optic neuropathy. J Med Genet. 1989 Dec;26(12):739–743. doi: 10.1136/jmg.26.12.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Bindoff L. A., McCullough D. A., Kubacka I., Poulton J., Mackey D., Taylor L., Turnbull D. M. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991 Nov;49(5):939–950. [PMC free article] [PubMed] [Google Scholar]
- Howell N. Evolutionary conservation of protein regions in the protonmotive cytochrome b and their possible roles in redox catalysis. J Mol Evol. 1989 Aug;29(2):157–169. doi: 10.1007/BF02100114. [DOI] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Xu M., McCullough D. A. Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. Am J Hum Genet. 1991 May;48(5):935–942. [PMC free article] [PubMed] [Google Scholar]
- Howell N., McCullough D. A., Kubacka I., Halvorson S., Mackey D. The sequence of human mtDNA: the question of errors versus polymorphisms. Am J Hum Genet. 1992 Jun;50(6):1333–1340. [PMC free article] [PubMed] [Google Scholar]
- Howell N., McCullough D. An example of Leber hereditary optic neuropathy not involving a mutation in the mitochondrial ND4 gene. Am J Hum Genet. 1990 Oct;47(4):629–634. [PMC free article] [PubMed] [Google Scholar]
- Huoponen K., Vilkki J., Aula P., Nikoskelainen E. K., Savontaus M. L. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1991 Jun;48(6):1147–1153. [PMC free article] [PubMed] [Google Scholar]
- Johns D. R., Berman J. Alternative, simultaneous complex I mitochondrial DNA mutations in Leber's hereditary optic neuropathy. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1324–1330. doi: 10.1016/0006-291x(91)91567-v. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Neufeld M. J. Cytochrome b mutations in Leber hereditary optic neuropathy. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1358–1364. doi: 10.1016/0006-291x(91)92088-2. [DOI] [PubMed] [Google Scholar]
- Lessell S., Gise R. L., Krohel G. B. Bilateral optic neuropathy with remission in young men. Variation on a theme by Leber? Arch Neurol. 1983 Jan;40(1):2–6. doi: 10.1001/archneur.1983.04050010022005. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Lin R., Wisniewski H. M., Hwang Y. W., Grundke-Iqbal I., Healy-Louie G., Iqbal K. Detection of point mutations in codon 331 of mitochondrial NADH dehydrogenase subunit 2 in Alzheimer's brains. Biochem Biophys Res Commun. 1992 Jan 15;182(1):238–246. doi: 10.1016/s0006-291x(05)80136-6. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E., Hoyt W. F., Nummelin K. Ophthalmoscopic findings in Leber's hereditary optic neuropathy. II. The fundus findings in the affected family members. Arch Ophthalmol. 1983 Jul;101(7):1059–1068. doi: 10.1001/archopht.1983.01040020061011. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E., Hoyt W. F., Nummelin K., Schatz H. Fundus findings in Leber's hereditary optic neuroretinopathy. III. Fluorescein angiographic studies. Arch Ophthalmol. 1984 Jul;102(7):981–989. doi: 10.1001/archopht.1984.01040030783017. [DOI] [PubMed] [Google Scholar]
- Noer A. S., Sudoyo H., Lertrit P., Thyagarajan D., Utthanaphol P., Kapsa R., Byrne E., Marzuki S. A tRNA(Lys) mutation in the mtDNA is the causal genetic lesion underlying myoclonic epilepsy and ragged-red fiber (MERRF) syndrome. Am J Hum Genet. 1991 Oct;49(4):715–722. [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Ino H., Ohno K., Sano T., Wada Y., Yoneda M., Tanno Y., Miyatake T., Tanaka T. Distinct clustering of point mutations in mitochondrial DNA among patients with mitochondrial encephalomyopathies and with Parkinson's disease. Biochem Biophys Res Commun. 1991 Apr 30;176(2):938–946. doi: 10.1016/s0006-291x(05)80276-1. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Hoyt W. F., Susac J. O. Ocular fundus in acute Leber optic neuropathy. Arch Ophthalmol. 1973 Nov;90(5):349–354. doi: 10.1001/archopht.1973.01000050351002. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]


