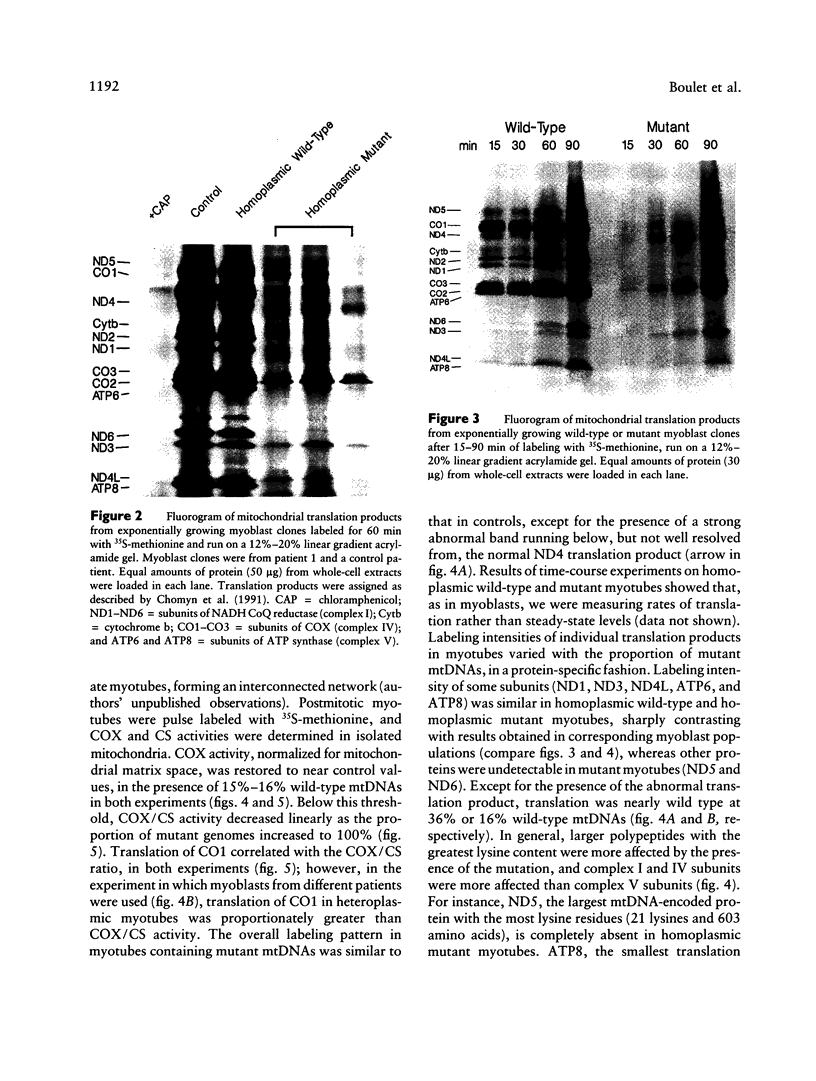
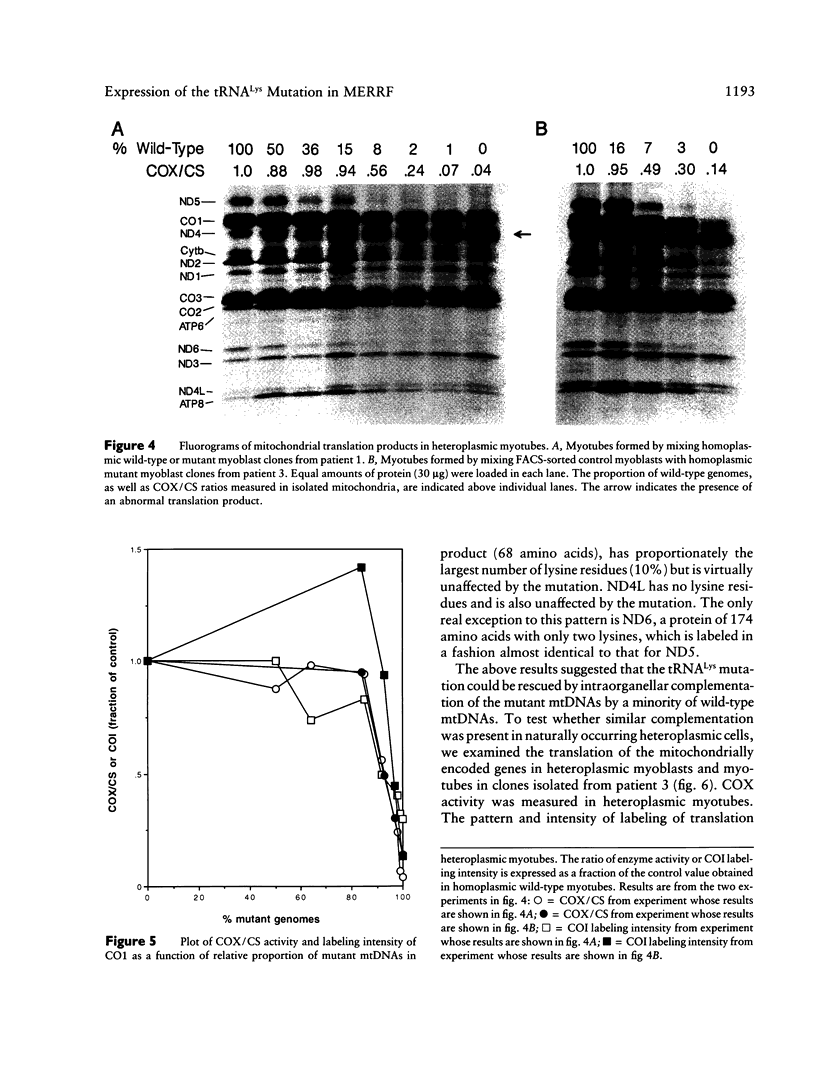
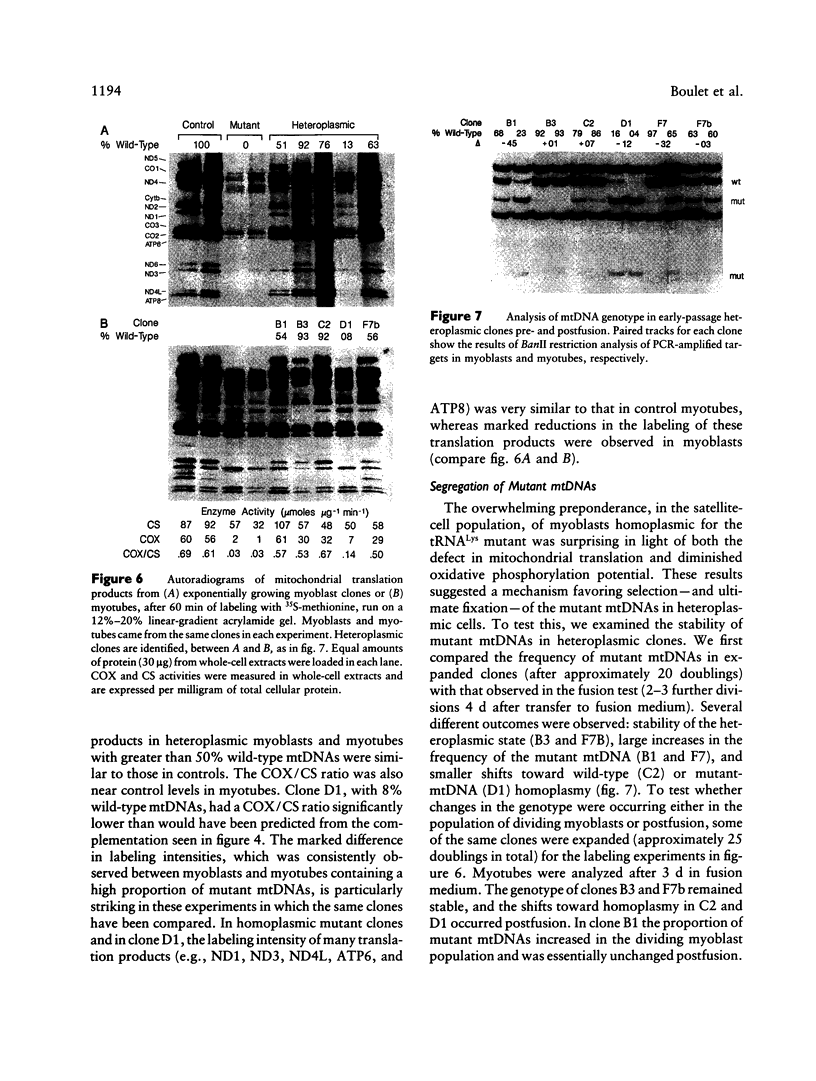
Abstract
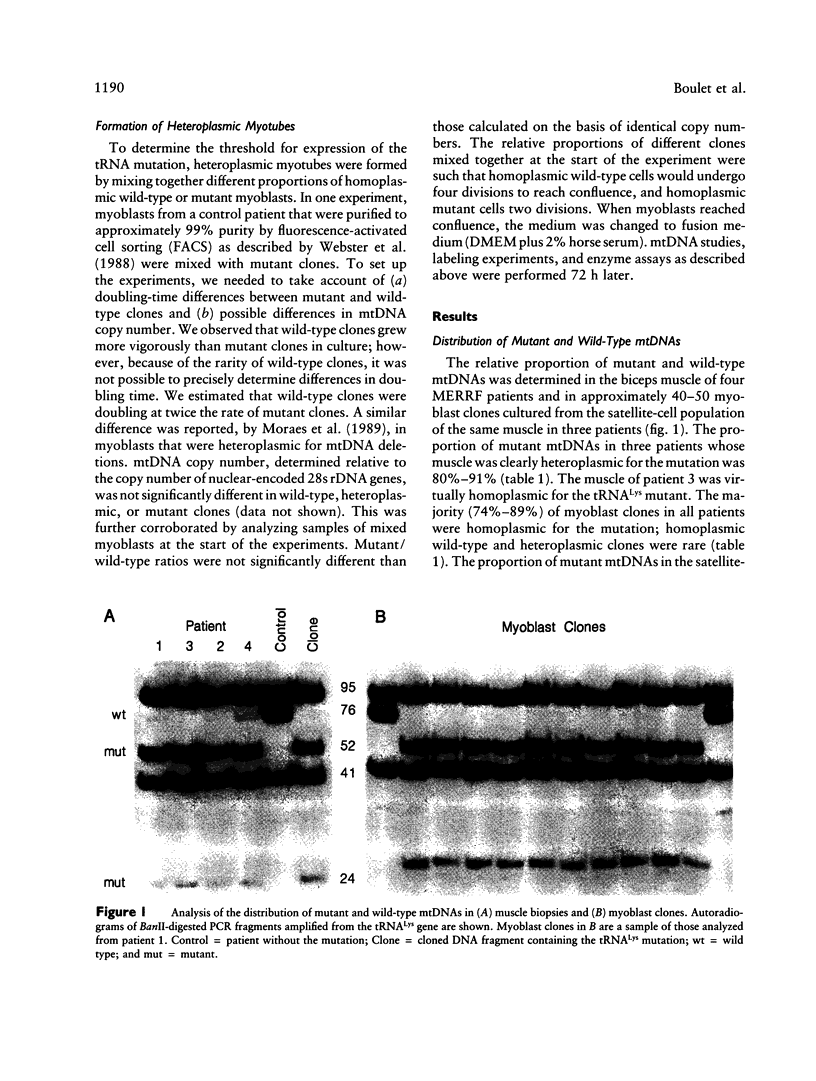
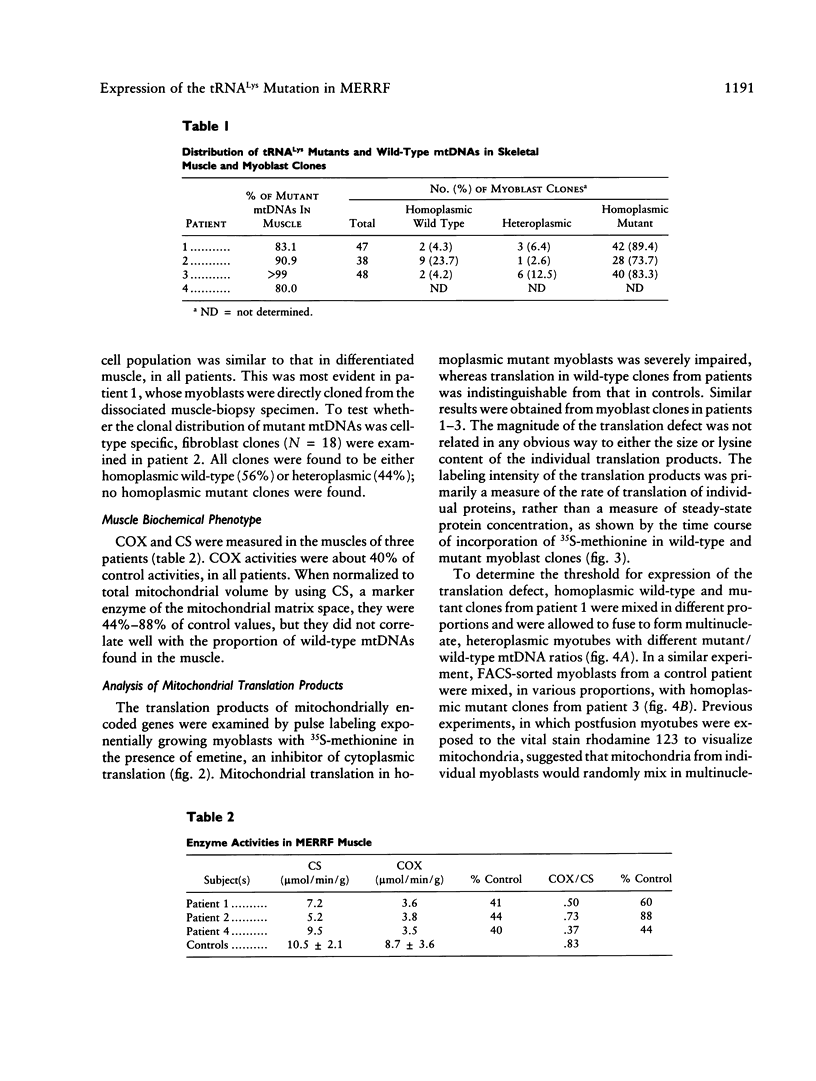
We investigated the distribution and expression of mutant mtDNAs carrying the A-to-G mutation at position 8344 in the tRNA(Lys) gene in the skeletal muscle of four patients with myoclonus epilepsy and ragged-red fibers (MERRF). The proportion of mutant genomes was greater than 80% of total mtDNAs in muscle samples of all patients and was associated with a decrease in the activity of cytochrome c oxidase (COX). The vast majority of myoblasts, cloned from the satellite-cell population in the same muscles, were homoplasmic for the mutation. The overall proportion of mutant mtDNAs in this population was similar to that in differentiated muscle, suggesting that the ratio of mutant to wild-type mtDNAs in skeletal muscle is determined either in the ovum or during early development and changes little with age. Translation of all mtDNA-encoded genes was severely depressed in homoplasmic mutant myoblast clones but not in heteroplasmic or wild-type clones. The threshold for biochemical expression of the mutation was determined in heteroplasmic myotubes formed by fusion of different proportions of mutant and wild-type myoblasts. The magnitude of the decrease in translation in myotubes containing mutant mtDNAs was protein specific. Complex I and IV subunits were more affected than complex V subunits, and there was a rough correlation with both protein size and number of lysine residues. Approximately 15% wild-type mtDNAs restored translation and COX activity to near normal levels. These results show that the A-to-G substitution in tRNA(Lys) is a functionally recessive mutation that can be rescued by intraorganellar complementation with a small proportion of wild-type mtDNAs and explain the steep threshold for expression of the MERRF clinical phenotype.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Ashley M. V., Laipis P. J., Hauswirth W. W. Rapid segregation of heteroplasmic bovine mitochondria. Nucleic Acids Res. 1989 Sep 25;17(18):7325–7331. doi: 10.1093/nar/17.18.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Ching E. Biogenesis of mitochondrial proteins in HeLa cells. Methods Enzymol. 1979;56:66–79. doi: 10.1016/0076-6879(79)56010-8. [DOI] [PubMed] [Google Scholar]
- Attardi G., Chomyn A., King M. P., Kruse B., Polosa P. L., Murdter N. N. Regulation of mitochondrial gene expression in mammalian cells. Biochem Soc Trans. 1990 Aug;18(4):509–513. doi: 10.1042/bst0180509. [DOI] [PubMed] [Google Scholar]
- Berkovic S. F., Shoubridge E. A., Andermann F., Andermann E., Carpenter S., Karpati G. Clinical spectrum of mitochondrial DNA mutation at base pair 8344. Lancet. 1991 Aug 17;338(8764):457–457. doi: 10.1016/0140-6736(91)91090-h. [DOI] [PubMed] [Google Scholar]
- Bindoff L. A., Desnuelle C., Birch-Machin M. A., Pellissier J. F., Serratrice G., Dravet C., Bureau M., Howell N., Turnbull D. M. Multiple defects of the mitochondrial respiratory chain in a mitochondrial encephalopathy (MERRF): a clinical, biochemical and molecular study. J Neurol Sci. 1991 Mar;102(1):17–24. doi: 10.1016/0022-510x(91)90088-o. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski M., Stepien G., Shoffner J. M., Lott M. T., Kanter K., Wallace D. C. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. JAMA. 1991 Oct 2;266(13):1812–1816. [PubMed] [Google Scholar]
- Fukuhara N., Tokiguchi S., Shirakawa K., Tsubaki T. Myoclonus epilepsy associated with ragged-red fibres (mitochondrial abnormalities ): disease entity or a syndrome? Light-and electron-microscopic studies of two cases and review of literature. J Neurol Sci. 1980 Jul;47(1):117–133. doi: 10.1016/0022-510x(80)90031-3. [DOI] [PubMed] [Google Scholar]
- Ham R. G., St Clair J. A., Webster C., Blau H. M. Improved media for normal human muscle satellite cells: serum-free clonal growth and enhanced growth with low serum. In Vitro Cell Dev Biol. 1988 Aug;24(8):833–844. doi: 10.1007/BF02623656. [DOI] [PubMed] [Google Scholar]
- Larsson N. G., Holme E., Kristiansson B., Oldfors A., Tulinius M. Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res. 1990 Aug;28(2):131–136. doi: 10.1203/00006450-199008000-00011. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka T., Goto Y., Yoneda M., Nonaka I. Muscle histopathology in myoclonus epilepsy with ragged-red fibers (MERRF). J Neurol Sci. 1991 Dec;106(2):193–198. doi: 10.1016/0022-510x(91)90257-8. [DOI] [PubMed] [Google Scholar]
- Michaels G. S., Hauswirth W. W., Laipis P. J. Mitochondrial DNA copy number in bovine oocytes and somatic cells. Dev Biol. 1982 Nov;94(1):246–251. doi: 10.1016/0012-1606(82)90088-4. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Schon E. A., DiMauro S., Miranda A. F. Heteroplasmy of mitochondrial genomes in clonal cultures from patients with Kearns-Sayre syndrome. Biochem Biophys Res Commun. 1989 Apr 28;160(2):765–771. doi: 10.1016/0006-291x(89)92499-6. [DOI] [PubMed] [Google Scholar]
- Noer A. S., Sudoyo H., Lertrit P., Thyagarajan D., Utthanaphol P., Kapsa R., Byrne E., Marzuki S. A tRNA(Lys) mutation in the mtDNA is the causal genetic lesion underlying myoclonic epilepsy and ragged-red fiber (MERRF) syndrome. Am J Hum Genet. 1991 Oct;49(4):715–722. [PMC free article] [PubMed] [Google Scholar]
- Oliver N. A., Wallace D. C. Assignment of two mitochondrially synthesized polypeptides to human mitochondrial DNA and their use in the study of intracellular mitochondrial interaction. Mol Cell Biol. 1982 Jan;2(1):30–41. doi: 10.1128/mcb.2.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Rosing H. S., Hopkins L. C., Wallace D. C., Epstein C. M., Weidenheim K. Maternally inherited mitochondrial myopathy and myoclonic epilepsy. Ann Neurol. 1985 Mar;17(3):228–237. doi: 10.1002/ana.410170303. [DOI] [PubMed] [Google Scholar]
- Seibel P., Degoul F., Bonne G., Romero N., François D., Paturneau-Jouas M., Ziegler F., Eymard B., Fardeau M., Marsac C. Genetic biochemical and pathophysiological characterization of a familial mitochondrial encephalomyopathy (MERRF). J Neurol Sci. 1991 Oct;105(2):217–224. doi: 10.1016/0022-510x(91)90148-z. [DOI] [PubMed] [Google Scholar]
- Shih K. D., Yen T. C., Pang C. Y., Wei Y. H. Mitochondrial DNA mutation in a Chinese family with myoclonic epilepsy and ragged-red fiber disease. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1109–1116. doi: 10.1016/0006-291x(91)91535-k. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Mitochondrial DNA in mortal and immortal human cells. Genome number, integrity, and methylation. J Biol Chem. 1983 Aug 10;258(15):9078–9085. [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Karpati G., Hastings K. E. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell. 1990 Jul 13;62(1):43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Jordan J. M., Vinograd J. In vivo effects of intercalating drugs on the superhelix density of mitochondrial DNA isolated from human and mouse cells in culture. J Mol Biol. 1971 Jul 28;59(2):255–272. doi: 10.1016/0022-2836(71)90050-7. [DOI] [PubMed] [Google Scholar]
- Tanno Y., Yoneda M., Nonaka I., Tanaka K., Miyatake T., Tsuji S. Quantitation of mitochondrial DNA carrying tRNALys mutation in MERRF patients. Biochem Biophys Res Commun. 1991 Sep 16;179(2):880–885. doi: 10.1016/0006-291x(91)91900-w. [DOI] [PubMed] [Google Scholar]
- Trounce I., Byrne E., Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989 Mar 25;1(8639):637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitotic segregation of mitochondrial DNAs in human cell hybrids and expression of chloramphenicol resistance. Somat Cell Mol Genet. 1986 Jan;12(1):41–49. doi: 10.1007/BF01560726. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Zheng X. X., Lott M. T., Shoffner J. M., Hodge J. A., Kelley R. I., Epstein C. M., Hopkins L. C. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988 Nov 18;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Amati P., Bresolin N., Antozzi C., Piccolo G., Toscano A., DiDonato S. Rapid detection of the A----G(8344) mutation of mtDNA in Italian families with myoclonus epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1991 Feb;48(2):203–211. [PMC free article] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]