Abstract
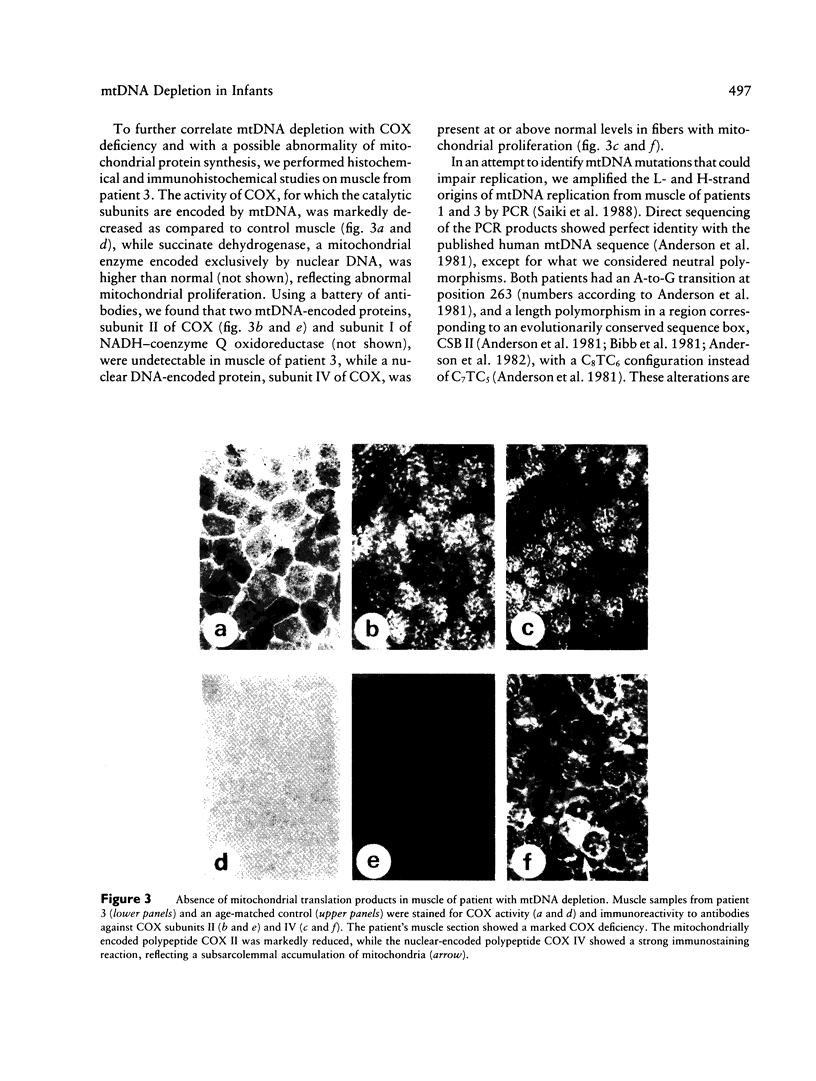
We studied two related infants with a fatal mitochondrial disease, affecting muscle in one and liver in the other. Quantitative analysis revealed a severe depletion of mtDNA in affected tissues. This genetic abnormality was also observed in muscle of an unrelated infant with myopathy and in muscle and kidney of a fourth child with myopathy and nephropathy. Biochemistry, immunohistochemistry, and in situ hybridization showed that the depletion of mtDNA in muscle fibers was correlated with a respiratory chain defect and with lack of mitochondrially translated proteins. Although the differential tissue involvement in these infants suggests mtDNA heteroplasmy, sequence analysis of mtDNA replication origins did not reveal any abnormality that could account for the low copy number.
Full text
PDF
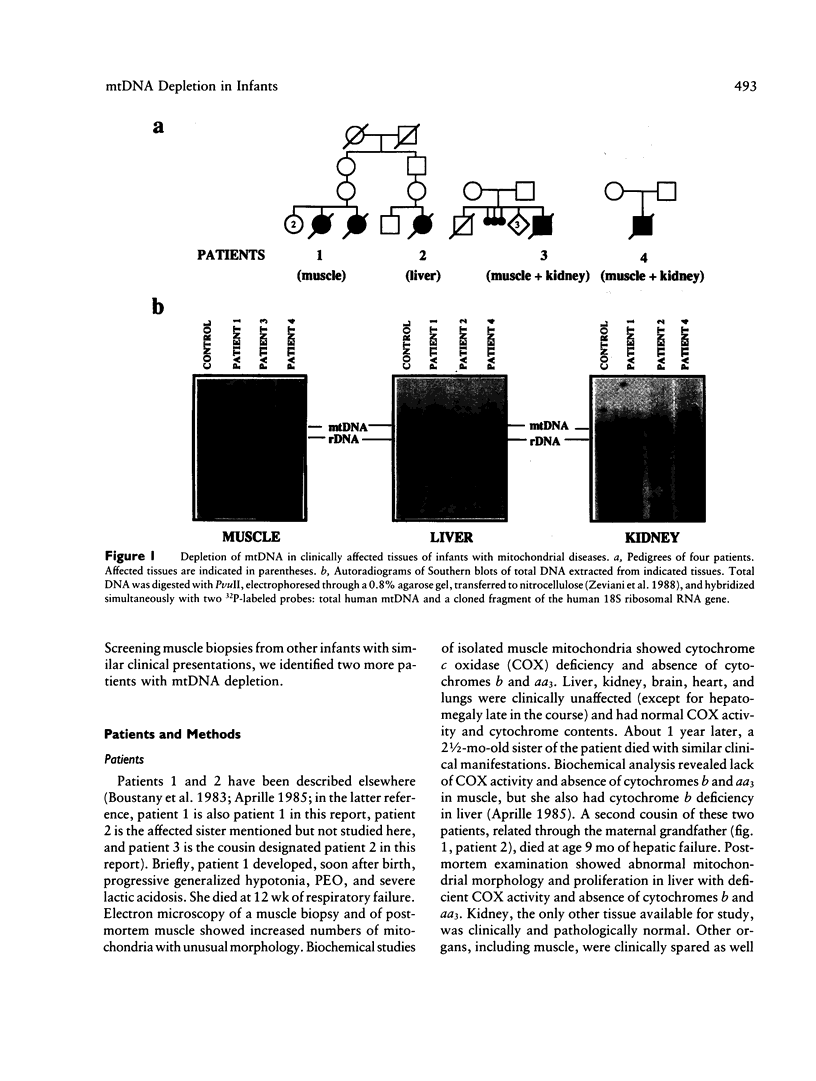

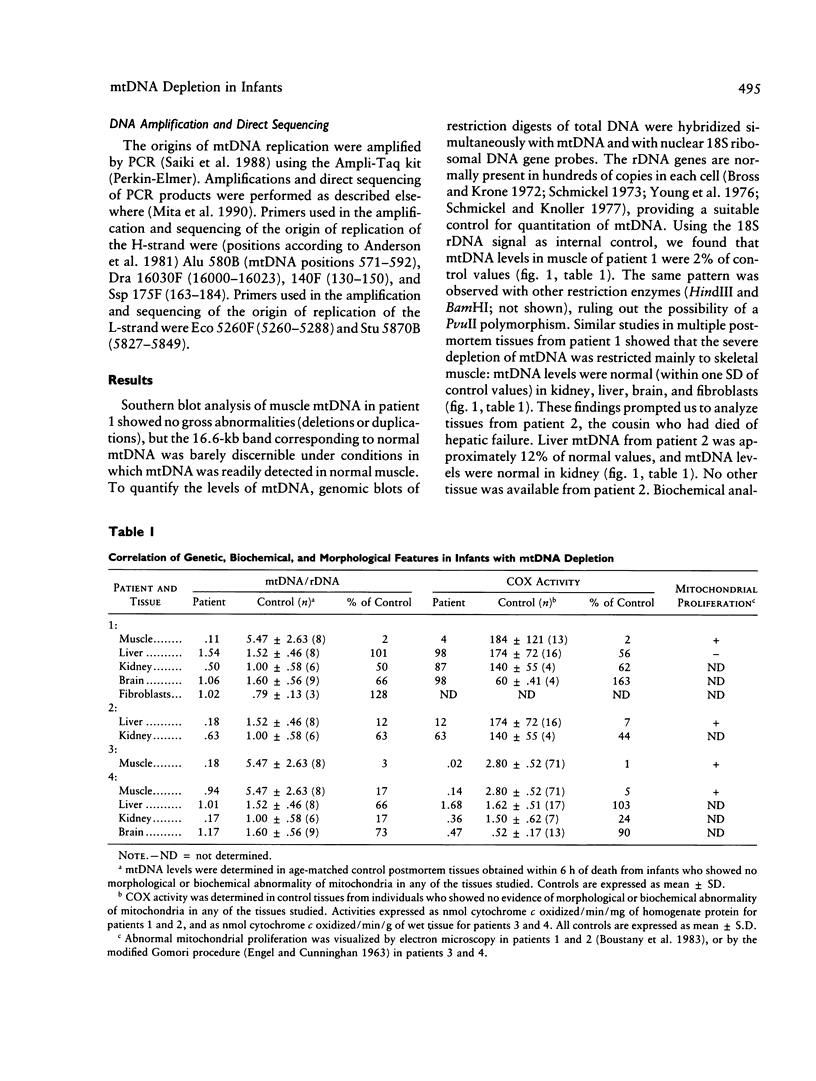
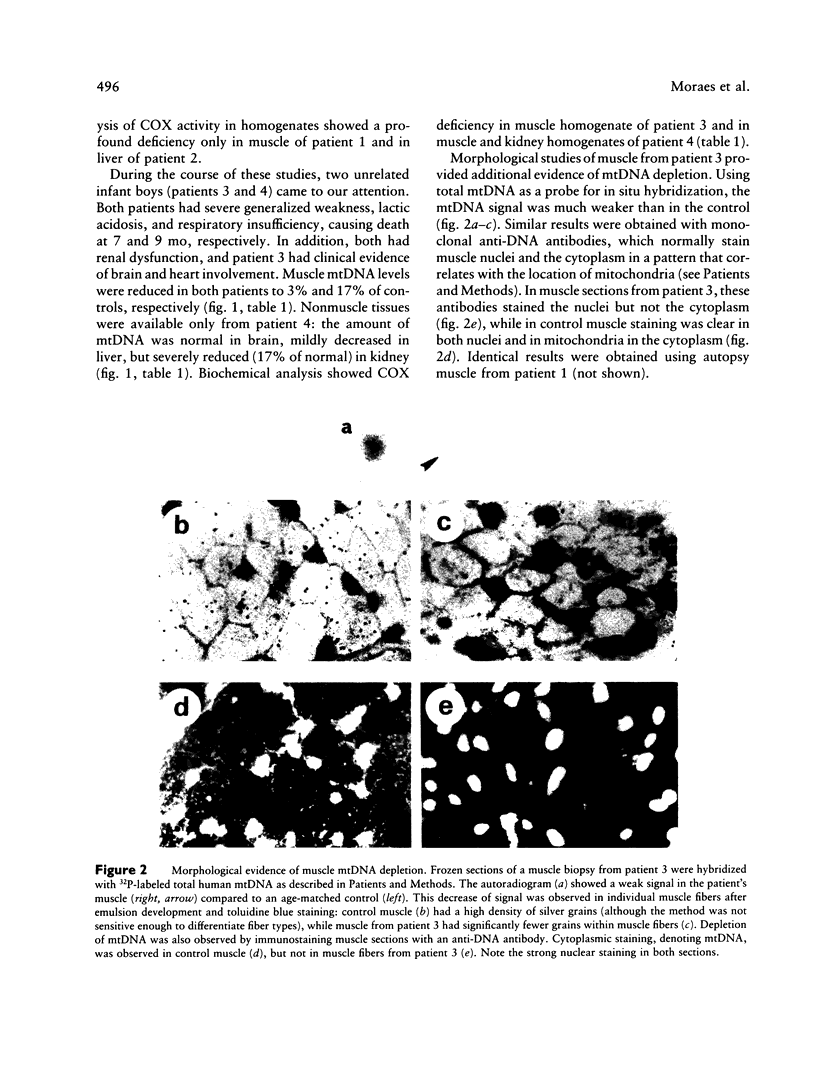




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Boustany R. N., Aprille J. R., Halperin J., Levy H., DeLong G. R. Mitochondrial cytochrome deficiency presenting as a myopathy with hypotonia, external ophthalmoplegia, and lactic acidosis in an infant and as fatal hepatopathy in a second cousin. Ann Neurol. 1983 Oct;14(4):462–470. doi: 10.1002/ana.410140411. [DOI] [PubMed] [Google Scholar]
- Bross K., Krone W. On the number of ribosomal RNA genes in man. Humangenetik. 1972;14(2):137–141. doi: 10.1007/BF00273298. [DOI] [PubMed] [Google Scholar]
- Cascio S. M., Wassarman P. M. Program of early development in the mammal: synthesis of mitochondrial proteins during oogenesis and early embryogenesis in the mouse. Dev Biol. 1981 Apr 15;83(1):166–172. doi: 10.1016/s0012-1606(81)80019-x. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Mouse RNAase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondrial RNA substrate. Cell. 1989 Jan 13;56(1):131–139. doi: 10.1016/0092-8674(89)90991-4. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Desjardins P., de Muys J. M., Morais R. An established avian fibroblast cell line without mitochondrial DNA. Somat Cell Mol Genet. 1986 Mar;12(2):133–139. doi: 10.1007/BF01560660. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Servidei S., Zeviani M., DiRocco M., DeVivo D. C., DiDonato S., Uziel G., Berry K., Hoganson G., Johnsen S. D. Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol. 1987 Oct;22(4):498–506. doi: 10.1002/ana.410220409. [DOI] [PubMed] [Google Scholar]
- ENGEL W. K., CUNNINGHAM G. G. RAPID EXAMINATION OF MUSCLE TISSUE. AN IMPROVED TRICHROME METHOD FOR FRESH-FROZEN BIOPSY SECTIONS. Neurology. 1963 Nov;13:919–923. doi: 10.1212/wnl.13.11.919. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Clayton D. A. Length heterogeneity of a conserved displacement-loop sequence in human mitochondrial DNA. Nucleic Acids Res. 1985 Nov 25;13(22):8093–8104. doi: 10.1093/nar/13.22.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Cooper J. M., Schapira A. H., Toscano A., Clark J. B., Morgan-Hughes J. A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989 Dec;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Michaels G. S., Hauswirth W. W., Laipis P. J. Mitochondrial DNA copy number in bovine oocytes and somatic cells. Dev Biol. 1982 Nov;94(1):246–251. doi: 10.1016/0012-1606(82)90088-4. [DOI] [PubMed] [Google Scholar]
- Mita S., Rizzuto R., Moraes C. T., Shanske S., Arnaudo E., Fabrizi G. M., Koga Y., DiMauro S., Schon E. A. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res. 1990 Feb 11;18(3):561–567. doi: 10.1093/nar/18.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Schmidt B., Schon E. A., DiMauro S., Bonilla E. Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9509–9513. doi: 10.1073/pnas.86.23.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Nelson I., Degoul F., Obermaier-Kusser B., Romero N., Borrone C., Marsac C., Vayssiere J. L., Gerbitz K., Fardeau M., Ponsot G. Mapping of heteroplasmic mitochondrial DNA deletions in Kearns-Sayre syndrome. Nucleic Acids Res. 1989 Oct 25;17(20):8117–8124. doi: 10.1093/nar/17.20.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikó L., Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol. 1976 Mar;49(1):1–10. doi: 10.1016/0012-1606(76)90253-0. [DOI] [PubMed] [Google Scholar]
- Pikó L. Synthesis of macromolecules in early mouse embryos cultured in vitro: RNA, DNA, and a polysaccharide component. Dev Biol. 1970 Feb;21(1):257–259. doi: 10.1016/0012-1606(70)90071-0. [DOI] [PubMed] [Google Scholar]
- Pikó L., Taylor K. D. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987 Oct;123(2):364–374. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Gardiner R. M. Duplications of mitochondrial DNA in mitochondrial myopathy. Lancet. 1989 Feb 4;1(8632):236–240. doi: 10.1016/s0140-6736(89)91256-7. [DOI] [PubMed] [Google Scholar]
- Robin E. D., Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol. 1988 Sep;136(3):507–513. doi: 10.1002/jcp.1041360316. [DOI] [PubMed] [Google Scholar]
- Rotig A., Colonna M., Bonnefont J. P., Blanche S., Fischer A., Saudubray J. M., Munnich A. Mitochondrial DNA deletion in Pearson's marrow/pancreas syndrome. Lancet. 1989 Apr 22;1(8643):902–903. doi: 10.1016/s0140-6736(89)92897-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schmickel R. D., Knoller M. Characterization and localization of the human genes for ribosomal ribonucleic acid. Pediatr Res. 1977 Aug;11(8):929–935. doi: 10.1203/00006450-197708000-00015. [DOI] [PubMed] [Google Scholar]
- Schmickel R. D. Quantitation of human ribosomal DNA: hybridization of human DNA with ribosomal RNA for quantitation and fractionation. Pediatr Res. 1973 Jan;7(1):5–12. doi: 10.1203/00006450-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Shanske S., Moraes C. T., Lombes A., Miranda A. F., Bonilla E., Lewis P., Whelan M. A., Ellsworth C. A., DiMauro S. Widespread tissue distribution of mitochondrial DNA deletions in Kearns-Sayre syndrome. Neurology. 1990 Jan;40(1):24–28. doi: 10.1212/wnl.40.1.24. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Mitochondrial DNA in mortal and immortal human cells. Genome number, integrity, and methylation. J Biol Chem. 1983 Aug 10;258(15):9078–9085. [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Wilson G. N., Hollar B. A., Waterson J. R., Schmickel R. D. Molecular analysis of cloned human 18S ribosomal DNA segments. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5367–5371. doi: 10.1073/pnas.75.11.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. D., Hell A., Birnie G. D. A new estimate of human ribosomal gene number. Biochim Biophys Acta. 1976 Dec 13;454(3):539–548. doi: 10.1016/0005-2787(76)90279-3. [DOI] [PubMed] [Google Scholar]
- Yuzaki M., Ohkoshi N., Kanazawa I., Kagawa Y., Ohta S. Multiple deletions in mitochondrial DNA at direct repeats of non-D-loop regions in cases of familial mitochondrial myopathy. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1352–1357. doi: 10.1016/0006-291x(89)91818-4. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Gellera C., Pannacci M., Uziel G., Prelle A., Servidei S., DiDonato S. Tissue distribution and transmission of mitochondrial DNA deletions in mitochondrial myopathies. Ann Neurol. 1990 Jul;28(1):94–97. doi: 10.1002/ana.410280118. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Servidei S., Gellera C., Bertini E., DiMauro S., DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989 May 25;339(6222):309–311. doi: 10.1038/339309a0. [DOI] [PubMed] [Google Scholar]