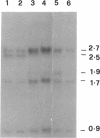
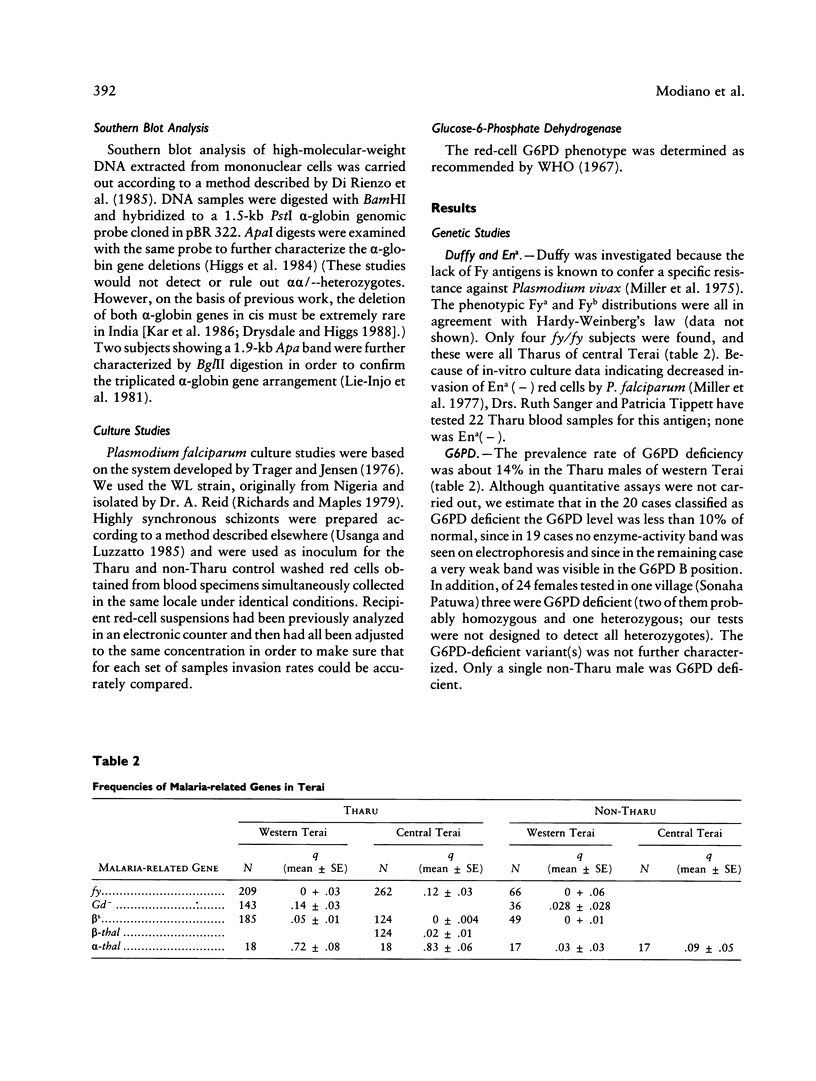
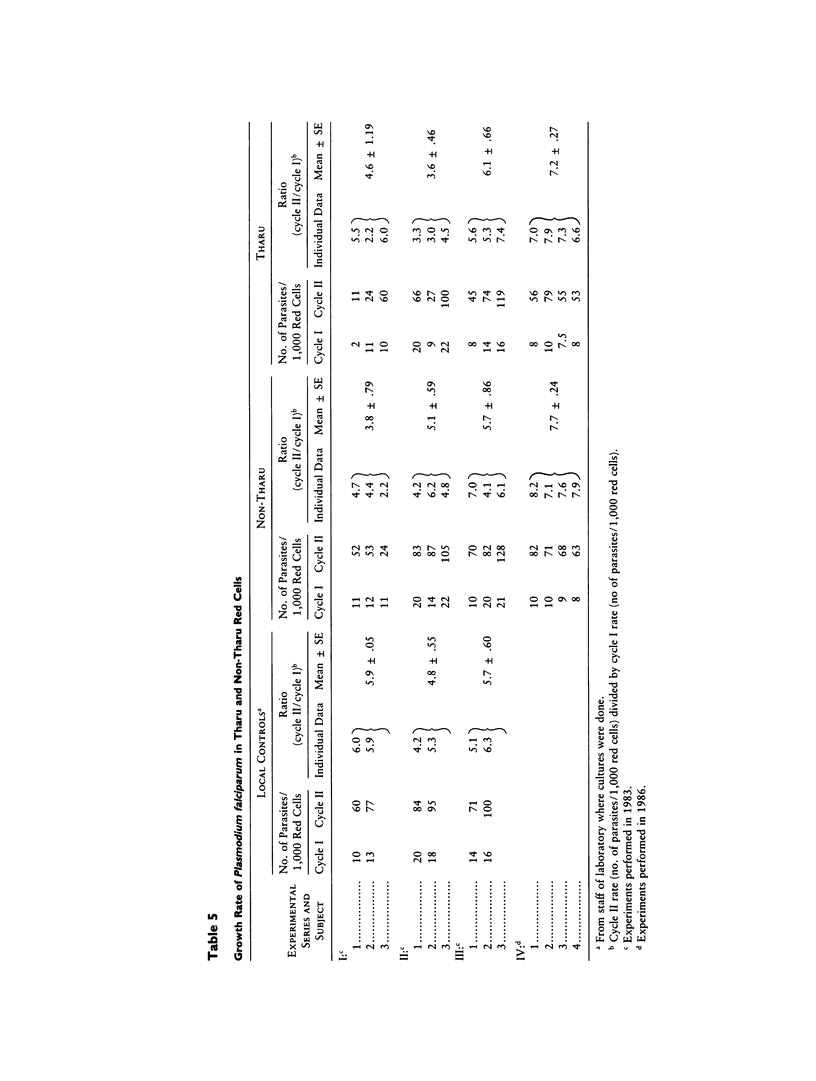
Abstract
We have previously reported that the Tharu people of the Terai region in southern Nepal have an incidence of malaria about sevenfold lower than that of synpatric non-Tharu people. In order to find out whether this marked resistance against malaria has a genetic basis, we have now determined in these populations the prevalence of candidate protective genes and have performed in-vitro cultures of Plasmodium falciparum in both Tharu and non-Tharu red cells. We have found significant but relatively low and variable frequencies of β-thal, βs, G6PD (−), and Duffy (a-b-) in different parts of the Terai region. The average in-vitro rate of invasion and of parasite multiplication did not differ significantly in red cells from Tharus versus those from non-Tharu controls. By contrast, the frequency of α-thalassemia is uniformly high in Tharus, with the majority of them having the homozygous α-/α-genotype and an overall α-thal gene (α-) frequency of .8. We suggest that holoendemic malaria has caused preferential survival of subjects with α-thal and that this genetic factor has enabled the Tharus as a population to survive for centuries in a malaria-holoendemic area. From our data we estimate that the α-thal homozygous state decreases morbidity from malaria by about 10-fold. This is an example of selection evolution toward fixation of an otherwise abnormal gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drysdale H. C., Higgs D. R. Alpha-thalassaemia in an Asian Indian. Br J Haematol. 1988 Feb;68(2):264–264. doi: 10.1111/j.1365-2141.1988.tb06201.x. [DOI] [PubMed] [Google Scholar]
- Efremov C. D., Huisman T. H., Bowman K., Wrightstone R. N., Shroeder W. A. Microchromatography of hemoglobins. II. A rapid method for the determination of hemoglobin A2. J Lab Clin Med. 1974 Apr;83(4):657–664. [PubMed] [Google Scholar]
- Embury S. H., Miller J. A., Dozy A. M., Kan Y. W., Chan V., Todd D. Two different molecular organizations account for the single alpha-globin gene of the alpha-thalassemia-2 genotype. J Clin Invest. 1980 Dec;66(6):1319–1325. doi: 10.1172/JCI109984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R., Losekoot M., van den Broek M. H., Oldenburg M., Rashida N., Schreuder A., Wijnen J. T., Giordano P. C., Nayudu N. V., Khan P. M. Prevalence and molecular heterogeneity of alfa+ thalassemia in two tribal populations from Andhra Pradesh, India. Hum Genet. 1988 Oct;80(2):157–160. doi: 10.1007/BF00702860. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Hill A. V., Bowden D. K., Weatherall D. J., Clegg J. B. Independent recombination events between the duplicated human alpha globin genes; implications for their concerted evolution. Nucleic Acids Res. 1984 Sep 25;12(18):6965–6977. doi: 10.1093/nar/12.18.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The population genetics of alpha-thalassemia and the malaria hypothesis. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):489–498. doi: 10.1101/sqb.1986.051.01.060. [DOI] [PubMed] [Google Scholar]
- Ifediba T. C., Stern A., Ibrahim A., Rieder R. F. Plasmodium falciparum in vitro: diminished growth in hemoglobin H disease erythrocytes. Blood. 1985 Feb;65(2):452–455. [PubMed] [Google Scholar]
- Kar B. C., Satapathy R. K., Kulozik A. E., Kulozik M., Sirr S., Serjeant B. E., Serjeant G. R. Sickle cell disease in Orissa State, India. Lancet. 1986 Nov 22;2(8517):1198–1201. doi: 10.1016/s0140-6736(86)92205-1. [DOI] [PubMed] [Google Scholar]
- Labie D., Srinivas R., Dunda O., Dode C., Lapoumeroulie C., Devi V., Devi S., Ramasami K., Elion J., Ducrocq R. Haplotypes in tribal Indians bearing the sickle gene: evidence for the unicentric origin of the beta S mutation and the unicentric origin of the tribal populations of India. Hum Biol. 1989 Aug;61(4):479–491. [PubMed] [Google Scholar]
- Lie-Injo L. E., Herrera A. R., Kan Y. W. Two types of triplicated alpha-globin loci in humans. Nucleic Acids Res. 1981 Aug 11;9(15):3707–3717. doi: 10.1093/nar/9.15.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Haynes J. D., McAuliffe F. M., Shiroishi T., Durocher J. R., McGinniss M. H. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977 Jul 1;146(1):277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Mason S. J., Dvorak J. A., McGinniss M. H., Rothman I. K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975 Aug 15;189(4202):561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- Nalbandian R. M., Nichols B. M., Camp F. R., Jr, Lusher J. M., Conte N. F., Henry R. L., Wolf P. L. Automated dithionite test for rapid, inexpensive detection of hemoglobin S and non-S sickling hemoglobinopathies. Clin Chem. 1971 Oct;17(10):1033–1037. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Ostrer H., Goff S. C., Sexton J. P. Abnormal RNA processing due to the exon mutation of beta E-globin gene. Nature. 1982 Dec 23;300(5894):768–769. doi: 10.1038/300768a0. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Wilson R. J. The interaction of malaria parasites with red blood cells. Br Med Bull. 1982 May;38(2):133–140. doi: 10.1093/oxfordjournals.bmb.a071749. [DOI] [PubMed] [Google Scholar]
- Richards W. H., Maples B. K. Studies on Plasmodium falciparum in continuous cultivation. I. The effect of chloroquine and pyrimethamine on parasite growth and viability. Ann Trop Med Parasitol. 1979 Apr;73(2):99–108. [PubMed] [Google Scholar]
- SMITHIES O. An improved procedure for starch-gel electrophoresis: further variations in the serum proteins of normal individuals. Biochem J. 1959 Mar;71(3):585–587. doi: 10.1042/bj0710585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S. L. Dynamics of malaria transmission with reference to development projects in Nepal. J Commun Dis. 1985 Dec;17(4):287–292. [PubMed] [Google Scholar]
- Terrenato L., Shrestha S., Dixit K. A., Luzzatto L., Modiano G., Morpurgo G., Arese P. Decreased malaria morbidity in the Tharu people compared to sympatric populations in Nepal. Ann Trop Med Parasitol. 1988 Feb;82(1):1–11. doi: 10.1080/00034983.1988.11812202. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Usanga E. A., Luzzatto L. Adaptation of Plasmodium falciparum to glucose 6-phosphate dehydrogenase-deficient host red cells by production of parasite-encoded enzyme. 1985 Feb 28-Mar 6Nature. 313(6005):793–795. doi: 10.1038/313793a0. [DOI] [PubMed] [Google Scholar]
- Yuthavong Y., Butthep P., Bunyaratvej A., Fucharoen S., Khusmith S. Impaired parasite growth and increased susceptibility to phagocytosis of Plasmodium falciparum infected alpha-thalassemia or hemoglobin Constant Spring red blood cells. Am J Clin Pathol. 1988 Apr;89(4):521–525. doi: 10.1093/ajcp/89.4.521. [DOI] [PubMed] [Google Scholar]