Abstract
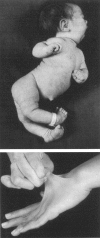
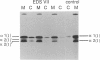
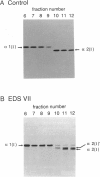
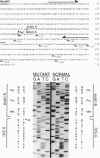
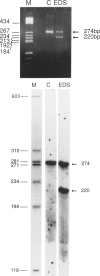
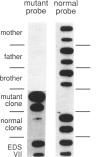
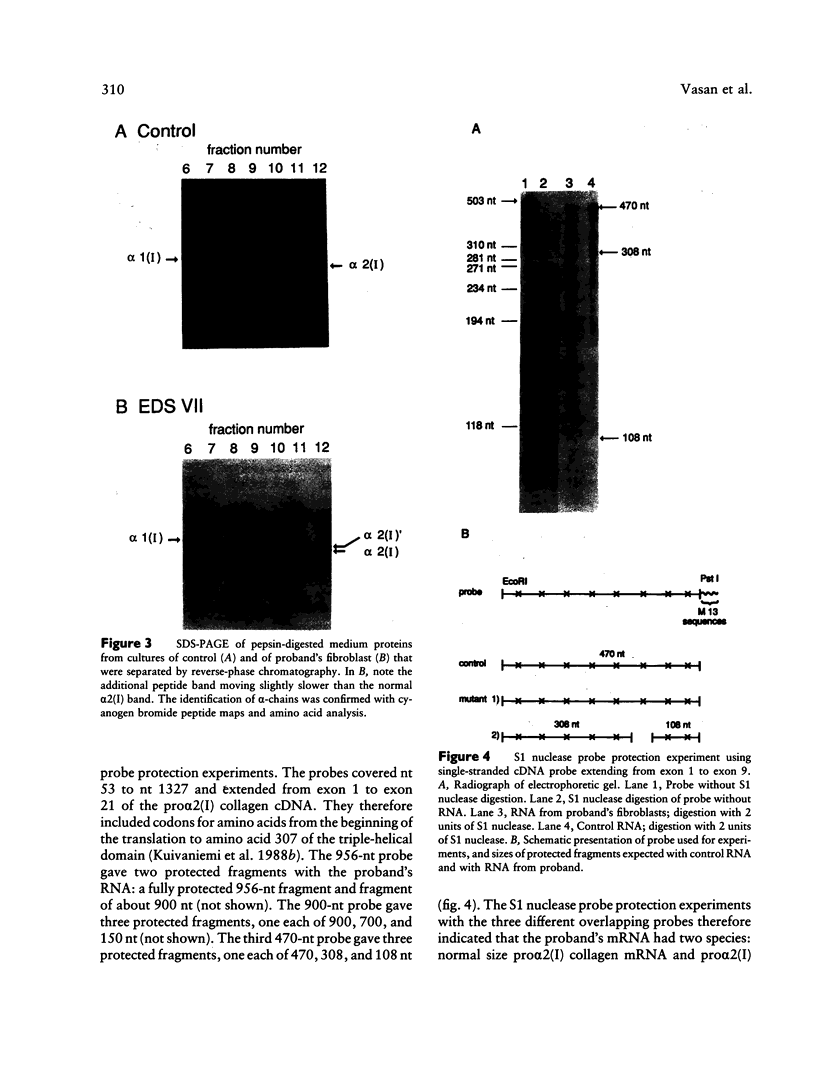
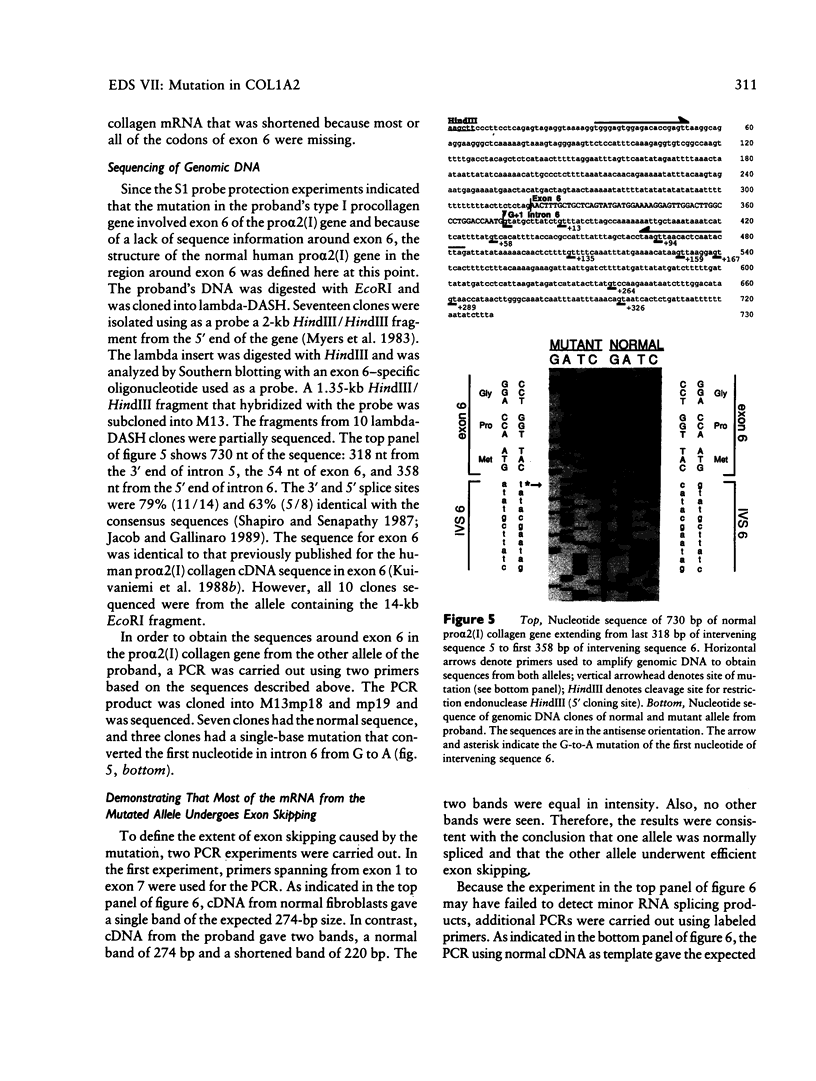
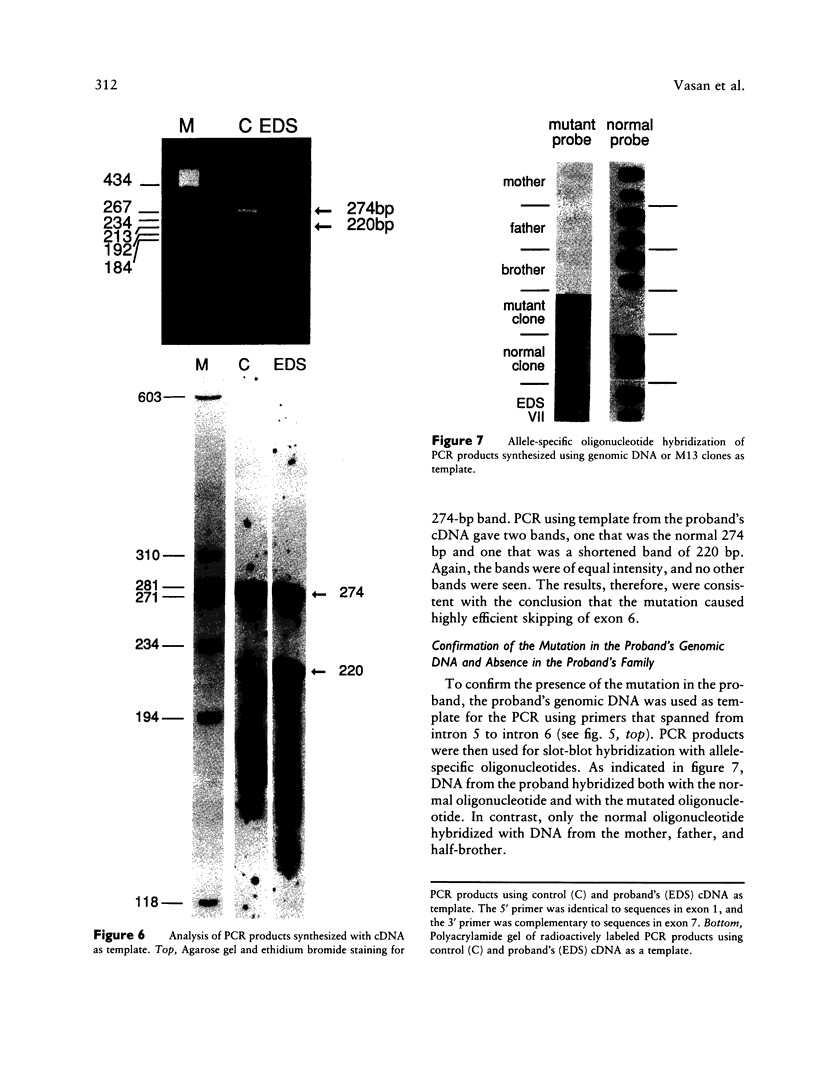
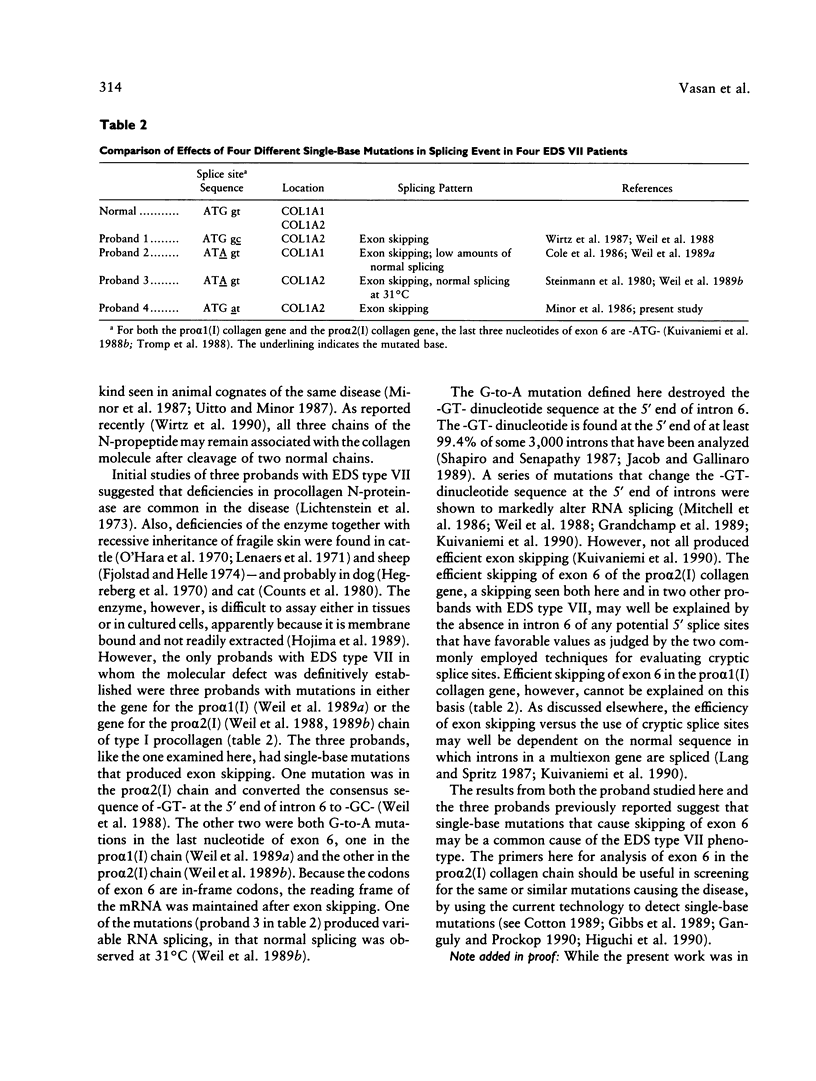
Fibroblasts from a proband with Ehlers-Danlos syndrome type VII synthesized approximately equal amounts of normal and shortened pro alpha 2(I) chains of type I procollagen. Nuclease S1 probe protection experiments with mRNA demonstrated that the pro alpha 2(I) chains were shortened because of a deletion of most or all of the 54 nucleotides in exon 6, the exon that contains codons for the cleavage site for procollagen N-proteinase. Sequencing of genomic clones revealed a single-base mutation that converted the first nucleotide of intron 6 from G to A. Therefore, the mutation was a change, in the -GT-consensus splice site, that produced efficient exon skipping. Allele-specific oligonucleotide hybridizations demonstrated that the proband's mother, father, and brother did not have the mutation. Therefore, the mutation was a sporadic one. Analysis of potential 5' splice sites in the 5' end of intron 6 indicated that none had favorable values by the two commonly employed techniques for evaluating such sites. The proband is the fourth reported proband with Ehlers-Danlos syndrome VII with a single-base mutation that causes skipping of exon 6 in the splicing of RNA from either the COL1A1 gene or COL1A2 gene. No other mutations in the two type I procollagen genes have been found in the syndrome. Therefore, such mutations may be a common cause of the phenotype. The primers developed should be useful in screening for the same or similar mutations causing the disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman J. F., Golub S. B. Assessment of procollagen processing defects by fibroblasts cultured in the presence of dextran sulphate. Biochem J. 1990 May 1;267(3):573–577. doi: 10.1042/bj2670573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton P., de Paepe A., Danks D., Finidori G., Gedde-Dahl T., Goodman R., Hall J. G., Hollister D. W., Horton W., McKusick V. A. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988 Mar;29(3):581–594. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- Børresen A. L., Berg K., Tsipouras P., Dickson L. A., Prockop D. J., Ramirez F. DNA polymorphisms in collagen genes: potential use in the study of disease. Prog Clin Biol Res. 1985;177:37–51. [PubMed] [Google Scholar]
- Chu M. L., de Wet W., Bernard M., Ding J. F., Morabito M., Myers J., Williams C., Ramirez F. Human pro alpha 1(I) collagen gene structure reveals evolutionary conservation of a pattern of introns and exons. 1984 Jul 26-Aug 1Nature. 310(5975):337–340. doi: 10.1038/310337a0. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Chan D., Chambers G. W., Walker I. D., Bateman J. F. Deletion of 24 amino acids from the pro-alpha 1(I) chain of type I procollagen in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1986 Apr 25;261(12):5496–5503. [PubMed] [Google Scholar]
- Cotton R. G. Detection of single base changes in nucleic acids. Biochem J. 1989 Oct 1;263(1):1–10. doi: 10.1042/bj2630001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts D. F., Byers P. H., Holbrook K. A., Hegreberg G. A. Dermatosparaxis in a Himalayan cat: I. Biochemical studies of dermal collagen. J Invest Dermatol. 1980 Feb;74(2):96–99. doi: 10.1111/1523-1747.ep12519991. [DOI] [PubMed] [Google Scholar]
- D'Alessio M., Bernard M., Pretorius P. J., de Wet W., Ramirez F., Pretorious P. J. Complete nucleotide sequence of the region encompassing the first twenty-five exons of the human pro alpha 1(I) collagen gene (COL1A1) Gene. 1988 Jul 15;67(1):105–115. doi: 10.1016/0378-1119(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Dickson L. A., de Wet W., Di Liberto M., Weil D., Ramirez F. Analysis of the promoter region and the N-propeptide domain of the human pro alpha 2(I) collagen gene. Nucleic Acids Res. 1985 May 24;13(10):3427–3438. doi: 10.1093/nar/13.10.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjolstad M., Helle O. A hereditary dysplasia of collagen tissues in sheep. J Pathol. 1974 Mar;112(3):183–188. doi: 10.1002/path.1711120309. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Prockop D. J. Detection of single-base mutations by reaction of DNA heteroduplexes with a water-soluble carbodiimide followed by primer extension: application to products from the polymerase chain reaction. Nucleic Acids Res. 1990 Jul 11;18(13):3933–3939. doi: 10.1093/nar/18.13.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Mignotte V., Wilson J. H., Te Velde K., Sandkuyl L., Roméo P. H., Goossens M., Nordmann Y. Tissue-specific splicing mutation in acute intermittent porphyria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):661–664. doi: 10.1073/pnas.86.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hegreberg G. A., Padgett G. A., Henson J. B. Connective tissue disease of dogs and mink resembling Ehlers-Danlos syndrome of man. 3. Histopathologic changes of the skin. Arch Pathol. 1970 Aug;90(2):159–166. [PubMed] [Google Scholar]
- Higuchi M., Wong C., Kochhan L., Olek K., Aronis S., Kasper C. K., Kazazian H. H., Jr, Antonarakis S. E. Characterization of mutations in the factor VIII gene by direct sequencing of amplified genomic DNA. Genomics. 1990 Jan;6(1):65–71. doi: 10.1016/0888-7543(90)90448-4. [DOI] [PubMed] [Google Scholar]
- Hojima Y., McKenzie J. A., van der Rest M., Prockop D. J. Type I procollagen N-proteinase from chick embryo tendons. Purification of a new 500-kDa form of the enzyme and identification of the catalytically active polypeptides. J Biol Chem. 1989 Jul 5;264(19):11336–11345. [PubMed] [Google Scholar]
- Hulmes D. J., Kadler K. E., Mould A. P., Hojima Y., Holmes D. F., Cummings C., Chapman J. A., Prockop D. J. Pleomorphism in type I collagen fibrils produced by persistence of the procollagen N-propeptide. J Mol Biol. 1989 Nov 20;210(2):337–345. doi: 10.1016/0022-2836(89)90335-5. [DOI] [PubMed] [Google Scholar]
- Jacob M., Gallinaro H. The 5' splice site: phylogenetic evolution and variable geometry of association with U1RNA. Nucleic Acids Res. 1989 Mar 25;17(6):2159–2180. doi: 10.1093/nar/17.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Ladda R. L., Prockop D. J. Inheritance of an RNA splicing mutation (G+ 1 IVS20) in the type III procollagen gene (COL3A1) in a family having aortic aneurysms and easy bruisability: phenotypic overlap between familial arterial aneurysms and Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1990 Jul;47(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Kontusaari S., Tromp G., Zhao M. J., Sabol C., Prockop D. J. Identical G+1 to A mutations in three different introns of the type III procollagen gene (COL3A1) produce different patterns of RNA splicing in three variants of Ehlers-Danlos syndrome. IV. An explanation for exon skipping some mutations and not others. J Biol Chem. 1990 Jul 15;265(20):12067–12074. [PubMed] [Google Scholar]
- Kuivaniemi H., Sabol C., Tromp G., Sippola-Thiele M., Prockop D. J. A 19-base pair deletion in the pro-alpha 2(I) gene of type I procollagen that causes in-frame RNA splicing from exon 10 to exon 12 in a proband with atypical osteogenesis imperfecta and in his asymptomatic mother. J Biol Chem. 1988 Aug 15;263(23):11407–11413. [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Chu M. L., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988 Jun 15;252(3):633–640. doi: 10.1042/bj2520633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang K. M., Spritz R. A. In vitro splicing pathways of pre-mRNAs containing multiple intervening sequences? Mol Cell Biol. 1987 Oct;7(10):3428–3437. doi: 10.1128/mcb.7.10.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Martin G. R., Kohn L. D., Byers P. H., McKusick V. A. Defect in conversion of procollagen to collagen in a form of Ehlers-Danlos syndrome. Science. 1973 Oct 19;182(4109):298–300. doi: 10.1126/science.182.4109.298. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Minor R. R., Sippola-Thiele M., McKeon J., Berger J., Prockop D. J. Defects in the processing of procollagen to collagen are demonstrable in cultured fibroblasts from patients with the Ehlers-Danlos and osteogenesis imperfecta syndromes. J Biol Chem. 1986 Jul 25;261(21):10006–10014. [PubMed] [Google Scholar]
- Mitchell P. J., Urlaub G., Chasin L. Spontaneous splicing mutations at the dihydrofolate reductase locus in Chinese hamster ovary cells. Mol Cell Biol. 1986 Jun;6(6):1926–1935. doi: 10.1128/mcb.6.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- O'Hara P. J., Read W. K., Romane W. M., Bridges C. H. A collagenous tissue dysplasia of calves. Lab Invest. 1970 Sep;23(3):307–314. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann B., Tuderman L., Peltonen L., Martin G. R., McKusick V. A., Prockop D. J. Evidence for a structural mutation of procollagen type I in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1980 Sep 25;255(18):8887–8893. [PubMed] [Google Scholar]
- Studencki A. B., Wallace R. B. Allele-specific hybridization using oligonucleotide probes of very high specific activity: discrimination of the human beta A- and beta S-globin genes. DNA. 1984;3(1):7–15. doi: 10.1089/dna.1.1984.3.7. [DOI] [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stacey A., Shikata H., Baldwin C. T., Jaenisch R., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 1(I) chain of human type I procollagen. Biochem J. 1988 Aug 1;253(3):919–922. doi: 10.1042/bj2530919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Myers J. C., Ramirez F., Prockop D. J. Restriction fragment length polymorphism associated with the pro alpha 2(I) gene of human type I procollagen. Application to a family with an autosomal dominant form of osteogenesis imperfecta. J Clin Invest. 1983 Oct;72(4):1262–1267. doi: 10.1172/JCI111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D., Bernard M., Combates N., Wirtz M. K., Hollister D. W., Steinmann B., Ramirez F. Identification of a mutation that causes exon skipping during collagen pre-mRNA splicing in an Ehlers-Danlos syndrome variant. J Biol Chem. 1988 Jun 25;263(18):8561–8564. [PubMed] [Google Scholar]
- Weil D., D'Alessio M., Ramirez F., Eyre D. R. Structural and functional characterization of a splicing mutation in the pro-alpha 2(I) collagen gene of an Ehlers-Danlos type VII patient. J Biol Chem. 1990 Sep 15;265(26):16007–16011. [PubMed] [Google Scholar]
- Weil D., D'Alessio M., Ramirez F., Steinmann B., Wirtz M. K., Glanville R. W., Hollister D. W. Temperature-dependent expression of a collagen splicing defect in the fibroblasts of a patient with Ehlers-Danlos syndrome type VII. J Biol Chem. 1989 Oct 5;264(28):16804–16809. [PubMed] [Google Scholar]
- Weil D., D'Alessio M., Ramirez F., de Wet W., Cole W. G., Chan D., Bateman J. F. A base substitution in the exon of a collagen gene causes alternative splicing and generates a structurally abnormal polypeptide in a patient with Ehlers-Danlos syndrome type VII. EMBO J. 1989 Jun;8(6):1705–1710. doi: 10.1002/j.1460-2075.1989.tb03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz M. K., Glanville R. W., Steinmann B., Rao V. H., Hollister D. W. Ehlers-Danlos syndrome type VIIB. Deletion of 18 amino acids comprising the N-telopeptide region of a pro-alpha 2(I) chain. J Biol Chem. 1987 Dec 5;262(34):16376–16385. [PubMed] [Google Scholar]
- Wirtz M. K., Keene D. R., Hori H., Glanville R. W., Steinmann B., Rao V. H., Hollister D. W. In vivo and in vitro noncovalent association of excised alpha 1 (I) amino-terminal propeptides with mutant pN alpha 2(I) collagen chains in native mutant collagen in a case of Ehlers-Danlos syndrome, type VII. J Biol Chem. 1990 Apr 15;265(11):6312–6317. [PubMed] [Google Scholar]
- de Wet W., Bernard M., Benson-Chanda V., Chu M. L., Dickson L., Weil D., Ramirez F. Organization of the human pro-alpha 2(I) collagen gene. J Biol Chem. 1987 Nov 25;262(33):16032–16036. [PubMed] [Google Scholar]