Abstract
The first component of the mitochondrial electron-transport chain is especially complex, consisting of 19 nuclear and seven mitochondrion-encoded subunits. Accordingly, a wide range of clinical manifestations are produced by the various mutations occurring in human populations. In this study, we analyze the subunit structure of complex I in fibroblasts from two patients who have distinct clinical phenotypes associated with complex I deficiency. The first patient died in the second week of life from overwhelming lactic acidosis. Severe complex I deficiency was evident in her fibroblasts, since alanine oxidation was markedly reduced whereas succinate oxidation was normal. Absence of a 20-kDa subunit was demonstrable when newly synthesized proteins were immunoprecipitated from pulse-labeled fibroblasts by anti-complex I antibody. Disordered assembly or decreased stability of the complex was suggested by deficiency of multiple subunits on Western immunoblots. The second patient exhibited a milder clinical phenotype, characterized by moderate lactic acidosis and developmental delay in childhood and by onset of seizures at 8 years of age. Oxidation studies demonstrated expression of the complex I deficiency in fibroblasts, but no subunit abnormalities were detected by immunoprecipitation or Western immunoblotting. This report demonstrates the utility of cultured fibroblasts in studying mutations affecting synthesis and assembly of complex I.
Full text
PDF

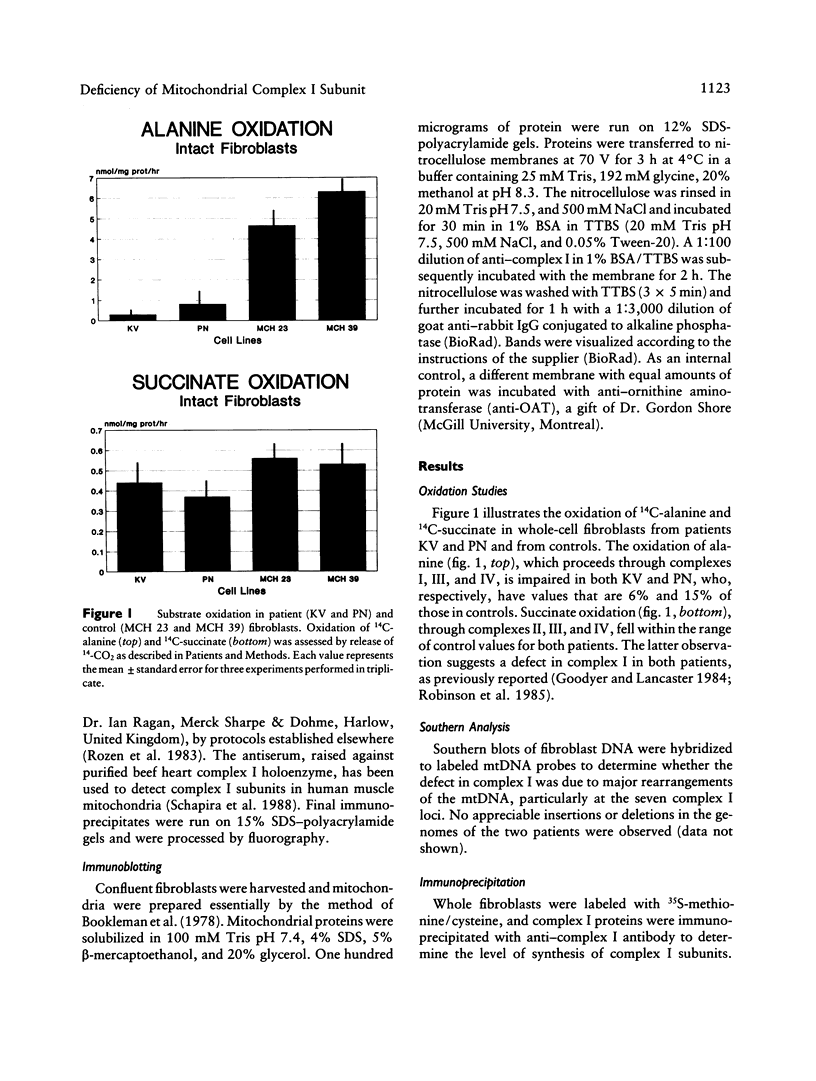
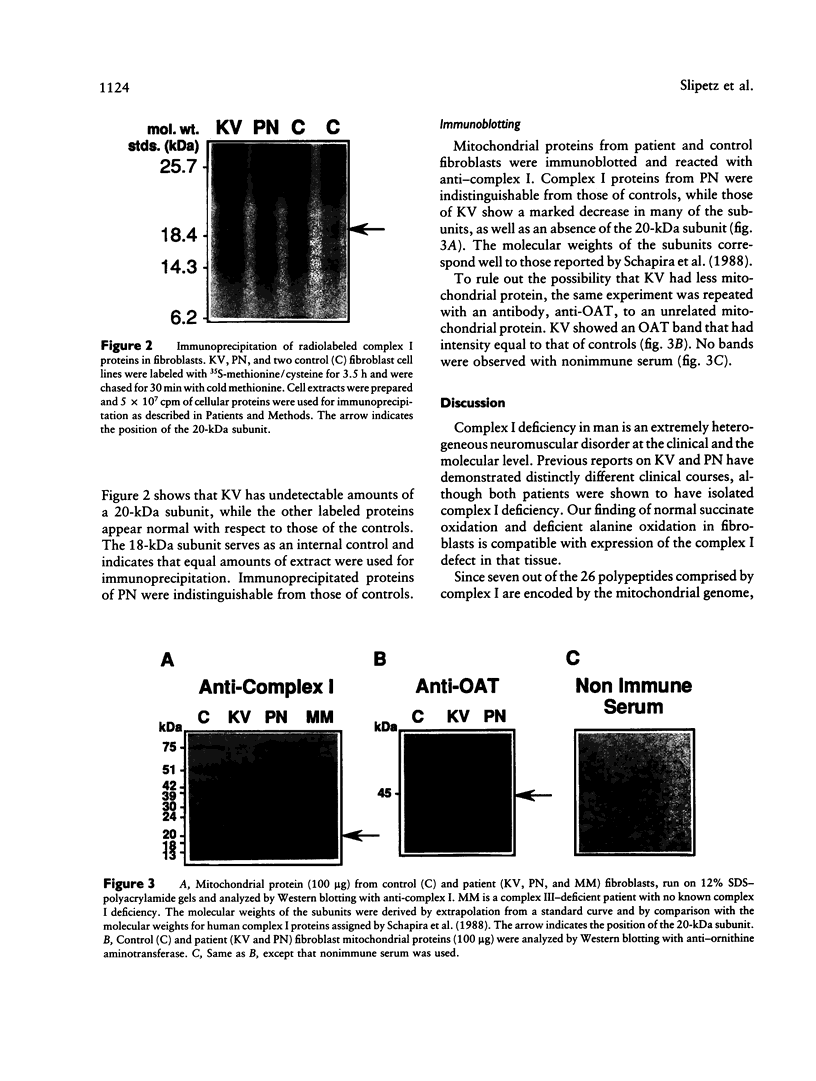


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Chomyn A., Doolittle R. F., Mariottini P., Ragan C. I. Seven unidentified reading frames of human mitochondrial DNA encode subunits of the respiratory chain NADH dehydrogenase. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):103–114. doi: 10.1101/sqb.1986.051.01.013. [DOI] [PubMed] [Google Scholar]
- Bookelman H., Trijbels J. M., Sengers R. C., Janssen A. J. Measurement of cytochromes in human skeletal muscle mitochondria, isolated from fresh and frozen stored muscle specimens. Biochem Med. 1978 Jun;19(3):366–373. doi: 10.1016/0006-2944(78)90037-6. [DOI] [PubMed] [Google Scholar]
- Booth F. A., Haworth J. C., Dilling L. A., Perry T. L., Greenberg C. R., Seargeant L. E., Penn A. M., Rhead W. J. Mitochondrial encephalomyopathy with associated aminoacidopathy in a male sibship. J Pediatr. 1989 Jul;115(1):81–88. doi: 10.1016/s0022-3476(89)80333-6. [DOI] [PubMed] [Google Scholar]
- Goodyer P. R., Lancaster G. A. Inherited lactic acidosis: correction of the defect in cultured fibroblasts. Pediatr Res. 1984 Nov;18(11):1144–1148. doi: 10.1203/00006450-198411000-00018. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L., Kerr D. S., Dahms B., Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987 Jul;80(1):71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki T., Tanaka M., Kobayashi M., Sugiyama N., Suzuki H., Nishikimi M., Ohnishi T., Nonaka I., Wada Y., Ozawa T. Disproportionate deficiency of iron-sulfur clusters and subunits of complex I in mitochondrial encephalomyopathy. Pediatr Res. 1989 Feb;25(2):194–201. doi: 10.1203/00006450-198902000-00023. [DOI] [PubMed] [Google Scholar]
- Ichiki T., Tanaka M., Nishikimi M., Suzuki H., Ozawa T., Kobayashi M., Wada Y. Deficiency of subunits of Complex I and mitochondrial encephalomyopathy. Ann Neurol. 1988 Mar;23(3):287–294. doi: 10.1002/ana.410230312. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Cleeter M. W., Ragan C. I., Batshaw M. L., Lehninger A. L. Congenital deficiency of two polypeptide subunits of the iron-protein fragment of mitochondrial complex I. J Clin Invest. 1987 Feb;79(2):463–467. doi: 10.1172/JCI112834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Schapira A. H., Cooper J. M., Clark J. B. Molecular defects of NADH-ubiquinone oxidoreductase (complex I) in mitochondrial diseases. J Bioenerg Biomembr. 1988 Jun;20(3):365–382. doi: 10.1007/BF00769638. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., De Meirleir L., Glerum M., Sherwood G., Becker L. Clinical presentation of mitochondrial respiratory chain defects in NADH-coenzyme Q reductase and cytochrome oxidase: clues to pathogenesis of Leigh disease. J Pediatr. 1987 Feb;110(2):216–222. doi: 10.1016/s0022-3476(87)80157-9. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., McKay N., Goodyer P., Lancaster G. Defective intramitochondrial NADH oxidation in skin fibroblasts from an infant with fatal neonatal lacticacidemia. Am J Hum Genet. 1985 Sep;37(5):938–946. [PMC free article] [PubMed] [Google Scholar]
- Rozen R., Fox J., Fenton W. A., Horwich A. L., Rosenberg L. E. Gene deletion and restriction fragment length polymorphisms at the human ornithine transcarbamylase locus. 1985 Feb 28-Mar 6Nature. 313(6005):815–817. doi: 10.1038/313815a0. [DOI] [PubMed] [Google Scholar]
- Rozen R., Noel C., Shore G. C. Effects of glucagon on biosynthesis of the mitochondrial enzyme, carbamoyl-phosphate synthase I, in primary hepatocytes and Morris hepatoma 5123D. Biochim Biophys Acta. 1983 Oct 13;741(1):47–54. doi: 10.1016/0167-4781(83)90008-8. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Morgan-Hughes J. A., Patel S. D., Cleeter M. J., Ragan C. I., Clark J. B. Molecular basis of mitochondrial myopathies: polypeptide analysis in complex-I deficiency. Lancet. 1988 Mar 5;1(8584):500–503. doi: 10.1016/s0140-6736(88)91296-2. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Nishikimi M., Suzuki H., Tada M., Ozawa T., Koga Y., Nonaka I. Deficiency of subunits of complex I or IV in mitochondrial myopathies: immunochemical and immunohistochemical study. J Inherit Metab Dis. 1987;10(3):284–288. doi: 10.1007/BF01800083. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Zheng X. X., Lott M. T., Shoffner J. M., Hodge J. A., Kelley R. I., Epstein C. M., Hopkins L. C. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988 Nov 18;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]




