Abstract
We suggest Milankovitch climate oscillations as a common cause for geographical patterns in species diversity, species' range sizes, polyploidy, and the degree of specialization and dispersability of organisms. Periodical changes in the orbit of the Earth cause climatic changes termed Milankovitch oscillations, leading to large changes in the size and location of species' geographical distributions. We name these recurrent changes “orbitally forced species' range dynamics” (ORD). The magnitude of ORD varies in space and time. ORD decreases gradual speciation (attained by gradual changes over many generations), increases range sizes and the proportions of species formed by polyploidy and other “abrupt” mechanisms, selects against specialization, and favor dispersability. Large ORD produces species prone neither to extinction nor gradual speciation. ORD increases with latitude. This produces latitudinal patterns, among them the gradient in species diversity and species' range sizes (Rapoport's rule). Differential ORD and its evolutionary consequences call for new conservation strategies on the regional to global scale.
Climate has fluctuated widely during the history of the Earth. Beyond seasonal variations, climatic variability increases in amplitude toward longer time scales, but have a marked peak on the time scale of 10–100 thousand years (kyr) caused by Milankovitch oscillations (1). Such are found during the entire Phanerozoic (2), and have varied in amplitude among geographical areas and geological epochs. The mean duration of a species in the fossil record varies among taxa from about 1 to 30 million years (3), implying that they possess properties that allow them to survive many Milankovitch oscillations.
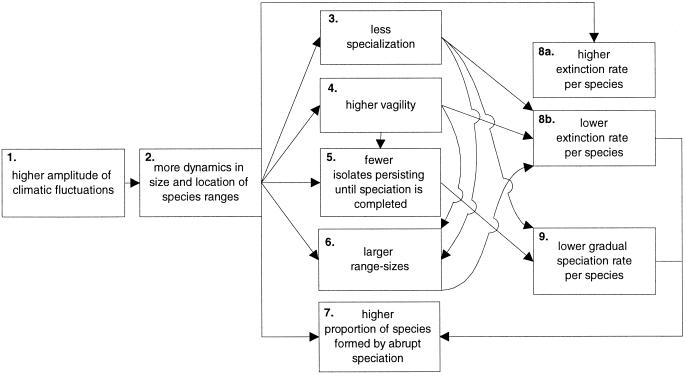
Milankovitch oscillations lead to large changes in the size and location of species' geographical distributions (4, 5). These orbitally forced species' range dynamics (ORD) can be seen as an overarching tier constraining evolutionary processes acting on shorter time scales (5). Adaptive changes may accumulate during the relatively short periods in between climatic shifts, but much of this is lost as populations go extinct or selection pressures are changed in conjunction with the next upheaval (5). Here, we systematically explore evolutionary consequences of ORD. We conclude that ORD decreases gradual speciation rates (as opposed to conventional wisdom), increases species' geographical ranges and the proportion of species formed by abrupt speciation, selects against specialization, and favors dispersability (Fig. 1). Large ORD leads to species prone neither to extinction nor gradual speciation. We suggest differential magnitudes of ORD as the common driving force explaining several poorly understood phenomena, e.g., latitudinal gradients in species diversity, range sizes, dispersability, specialization, and polyploidy. No previously suggested driving force explains all these phenomena. We explore the consequences of ORD in the context of latitudinal gradients, and then apply them to other geographical contrasts and long-term temporal patterns.
Figure 1.
Evolutionary consequences of ORD. Larger climatic oscillations at Milankovitch time scales (10–100 kyr) force more range dynamics. This selects against specialization and favors vagility (i.e., low dispersal ability and propensity), and causes lower gradual speciation rates, larger range sizes, and higher proportions of species formed by abrupt speciation (polyploidy). Larger ORD lowers gradual speciation rates, but affects extinction rates in both directions.
Climatic Oscillations and Species' Ranges.
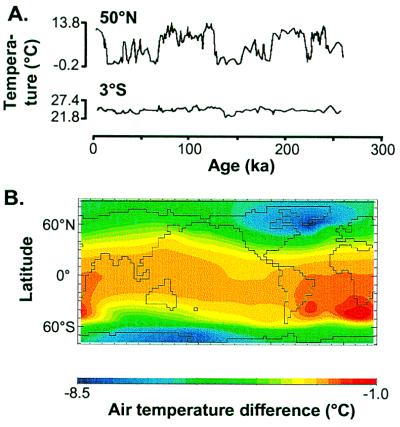
The obliquity of the axis of the Earth and the eccentricity of the orbit vary with 41- and 100-kyr periods, respectively, and the annual timing of the minimum Earth-sun distance varies with a 21-kyr period (1). These orbital oscillations cause variation in insolation that, combined with earthbound feedbacks, produce large and rapid changes in temperature and precipitation (4, 6) (Fig. 2A). The 41- and 100-kyr oscillations cause larger temperature changes toward the poles (6–8). Because changes in precipitation during the last 18 kyr were not correlated with latitude (7), we restrict our discussion on latitudinal gradients to the effects of temperature variability. Quaternary sea-surface temperature variations increase toward the poles (4, 6, 9), as does the difference in air temperatures between the last glacial maximum and the present (Fig. 2B). Although continental glacial climates in the tropics were up to 5°C cooler than at present (10), temperatures at high northern latitudes were up to 30°C lower (7).
Figure 2.
The amplitude of climatic oscillations is higher toward higher latitudes. (A) Variation in sea-surface temperatures (cold season) at two sites situated 50°N and 3°S in the Atlantic. Adapted from Imbrie et al. (6). (B) Difference in the mean annual air temperature between the last glacial maximum and the present, estimated from a recent general circulation model (9).
The larger temperature changes toward the poles caused more ORD both during the Quaternary (5) and before (11). During the last glacial cycle, the ranges of many north-temperate tree species contracted and expanded thousands of kilometers (12, 13). Insect species have also altered their geographical ranges enormously. Coope (14) found insect fossils in Britain from the last glacial, of species with present ranges restricted to the Tibetan plateau or eastern Siberia. ORD has been much smaller in tropical areas. Paleoecological studies show that rainforest plant taxa persisted locally during the last glacial in, e.g., Panama, (15) and in both the Andean foothill (16) and lowland Amazonia (17). Steep gradients such as mountain slopes reduce the need for distributional changes because species can survive by moving short distances along the gradient (18).
Climatic Oscillations Produce Vagile and Generalized Organisms.
Climatic oscillations select for vagility (dispersal ability and propensity) and generalism (Fig. 1, Boxes 3 and 4). Organisms must be vagile enough to track their moving habitat. For example, in lodge-pole pine Pinus contorta, which is expanding northwards, seed dispersability is highest in recently founded populations, suggesting ongoing selection for dispersal (19). Organisms with low specialization suffer smaller risks that their niches disappear completely. Moreover, they do not need to disperse as rapidly to track their habitat and may even survive locally. They are also more likely to survive while moving through heterogeneous environments. Climatic as well as range shifts place the organisms in new abiotic and biotic settings (5), leading to variable selection pressures over time, preventing high specialization. ORD also cause specialized populations to go extinct or come in contact and interbreed, thereby losing their specialization (20). The corollary of all these mechanisms is few specialized adaptations to contemporary environments in regions with large ORD. In contrast, in the climatically stable Hawaiian Islands, cricket populations have become extremely specialized to specific humidity and temperature conditions. The high specialization is interpreted as a result of the long-lived local populations (21).
The effects of ORD on specialization and vagility are well illustrated by alpine carabid beetles in Scandinavia, a region with large ORD. Alpine environments generally select against wings in insects, but alpine specialist species of Scandinavia are instead better flyers than species occurring in both alpine and lowland areas. Probably, only the good flyers among the alpine specialists were able to colonize from glacial refugia during the rapid postglacial warming, whereas flightless habitat generalists have been able to disperse slowly through interjacent habitats (22).
Specialization and Vagility vs. Latitude.
The greater ORD toward high latitudes should lead to stronger selection for generalism and vagility, producing latitudinal gradients in these properties. Indeed, low specialization and high vagility are typical attributes of high-latitude organisms.
Tropical animals are generally more specialized in their feeding (23–25). The number of habitats occupied by a mammal species increases with latitude more rapidly than expected from a null model (26). In the arctic tundra, many plant and animal species are more or less ubiquitous and vegetation types are often distinguished only by differences in the proportions of constituent species. There are, for example, very few plant species restricted to serpentine soils at high latitudes whereas this is common in the tropics (27).
Vagility increases with latitude in a wide range of taxa. A small plant or fungal diaspore is more readily dispersed than a large one. Small diaspores are also produced in higher numbers with similar input of resources and more diaspores mean longer maximum dispersal distance. Seed mass decreases with latitude in both intra- and intercontinental data sets and independent of dispersal mode, plant growth form, and phylogenetic relationships (28). Tropical lichens have fewer mechanisms of dispersal and larger spores, and tropical polypore fungi have larger spores than temperate ones (29–31). Tropical bird species are more sedentary than temperate ones. There are hundreds, perhaps thousands of tropical bird species living in coastal lowlands, which never have been recorded on any island lacking a Pleistocene land bridge, not even islands only a few km offshore (32). In contrast, the avifauna of temperate coastal islands is almost identical to the fauna of an equal area of the mainland. The larger seasonal variations in food availability at high latitudes contribute to this gradient in birds. In a few marine invertebrate taxa, the proportion of species having vagile pelagic larvae is lower at high latitudes (33).
Gradual Speciation.
Gradual speciation refers to the gradual evolution of reproductive barriers between populations over many generations, both in geographical and sympatric speciation, and irrespective of type of selection. Instantaneous or abrupt speciation causes immediate reproductive isolation through chromosomal events, e.g., polyploidy (34). For gradual speciation to occur, populations must be sufficiently isolated (geographically or ecologically) to prevent gene flow exceeding the differentiation rate of reproductive characters. Moreover, the isolation as well as the differentiating populations themselves (the incipient species) must persist during the time needed to evolve reproductive barriers (35). This takes tens of thousands to millions of years (34, 36, 37), although occurring much faster during adaptive radiations into large underexploited niches, such as in whitefish, sticklebacks (38), and cichlids.
Under stable conditions, natural selection against dispersal is often strong because most organisms are better adapted to the local conditions than environments elsewhere, and mortality during dispersal is usually high. Selection against mating between individuals specialized to different local environments can also be strong. These processes lead to low gene flow. If gene flow gets very low, gradual speciation becomes rampant, subdividing species into new ones with progressively smaller distributions, lower vagility, and/or higher specialization.
What then curbs gradual speciation in most of the world? Probably ORD. Much attention has been paid to different ways in which populations may become isolated and diverge, whereas the persistence part of the speciation process has been comparatively neglected (35). Hence, ORD have often been suggested to promote speciation by creating isolates. Instead, we argue that ORD affect regional differences in speciation rates more by its negative influence on persistence of incipient species than by its positive influence on rate of isolate formation (Fig. 1, Box 5). The short stable periods during Milankovitch oscillations are generally not long enough for gradual speciation to be completed before a new climatic shift reconnects the isolate with the main population (14, 20) or the incipient species go extinct (13, 39). Alluding to Darwin who used “permanent varieties” for species, there is not enough time for the varieties to become permanent. By an analogous argument, Diamond explained patterns in island endemism in land birds (32). Small islands or archipelagos with high population extinction rates produce fewer new species because most isolated populations do not persist until reproductive barriers have evolved.
There are many empirical examples of the negative effect of ORD on gradual speciation. In the African Lake Turkana basin, several molluscan populations diverged morphologically during Pleistocene periods of isolation (regressions), but these went extinct and were replaced with mollusks invading from the main populations during subsequent transgressions (40). Three-spined sticklebacks (Gasterosteus sp.) are marine species rapidly producing numerous diverging freshwater populations that probably go extinct (41) as their habitats disappear because of climatic changes. In Indopacific shallow water corals, the frequent sea level changes during the Quaternary repeatedly laid the continental shelves dry, so that any particular area was available for coral growth for on average only 3.2 kyr at a time. These frequent large-scale distributional changes probably prevented speciation, and most of the coral species have little geographical genetic subdivision (42). In Afrotropical birds and plants, low climatic amplitude, inferred from the occurrence of paleoendemic species that are extinct elsewhere, is well correlated with recently formed neoendemic species (43). In European trees, both extinction of isolates and broken isolation have prevented gradual speciation. Diverging northern populations of most European forest trees went extinct in situ at the end of interglacials (13). In the beginning of interglacials, Northern Europe was colonized from southeastern and southern refugia (12, 13). The European silver fir Abies alba formed five isolated populations during the last glacial. Three then expanded their ranges, came into contact, and mixed (44).
The larger ORD at high latitudes reduces the probability of isolates persisting until reproductive barriers have evolved (45, 46), thus reducing gradual speciation rates. This is strongly supported by the lack of endemic species on high-latitude islands (47). As in European trees, the extensive plant migrations during the Quaternary in most mountains of Middle Asia prevented the formation of new endemic species (48). In contrast, in Panama, the close-lying eastern and western mountain floras never merged during the last glacial, and the number of species endemic to either of the areas is high (15).
Vagility and Generalism Reduce Extinction and Gradual Speciation.
ORD increases the risk for extinction because organisms may fail to disperse or their habitats may become rare or disappear (Fig. 1, Box 8a). However, ORD also selects for generalism and vagility, thereby mitigating extinction in the long run (Fig. 1, Box 8b). Generalism and high vagility promote high regional population densities and wide distributions, and all four factors are correlated to low extinction rates (32, 49). The large potential of selection for generalism and vagility is shown by the large climatic oscillations of the Quaternary causing very few global extinctions in the oceans (50) and among terrestrial beetles (14).
High vagility and low specialization should also slow gradual speciation rates. High vagility enhances gene flow, making speciation less probable (34, 51) (Fig. 1, Boxes 4 and 5) and empirically, generalist clades speciate less than specialist ones (51–53) (Fig. 1, Boxes 3 and 9). Many marine taxa have high fecundity and highly vagile larvae, and these traits are associated with low speciation rates in the fossil record (49, 51). The same pattern is found among land plants (51, 54). Only sedentary taxa are able to radiate on small islands (55). In Hawaiian crickets, the low vagility and high specialization compared with their continental relatives have lead to a tremendous radiation resulting in a unique concentration of species (21). However, an organism must be vagile and generalized enough to colonize persistent habitat or real islands in the first place. By using a vertebrate data set, Lynch (56) argued that such peripatric speciation, although common on oceanic islands, is rare on continents.
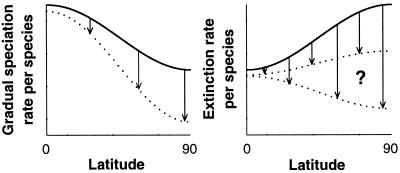
Thus, large ORD should produce species prone neither to extinction nor gradual speciation. Because high vagility and low specialization are typical characters of high-latitude species, the ORD-induced latitudinal gradient in gradual speciation rate is accentuated, whereas the gradient in extinction rate is reduced or may even become reversed (Fig. 3).
Figure 3.
Differential degrees of ORD generate a latitudinal gradient in gradual speciation rates. Larger ORD toward the poles reduces the gradual speciation rate but elevates the extinction rate (solid lines). However, ORD selects for vagility and against specialization, thereby lowering both speciation and extinction rates (arrows and dotted lines). Extinction is also lowered by the larger geographical ranges caused by ORD. Stronger rate reductions at high latitudes (longer arrows) accentuate the gradient in speciation rate, whereas the gradient in extinction rate is reduced or may even become reversed.
The Tropics—Cradle or Museum?
The latitudinal diversity gradient is ancient (57, 58) and valid for most higher taxa. The higher regional richness of species in the tropics is explained by higher diversification rates (59), resulting from higher speciation rates and/or lower extinction rates. Paleontological data are scarce, but a survey of studies shows that the mean age of taxa (mostly genera but also species and families) in most extant and fossil tropical faunas is lower, and in no case higher than high-latitude faunas (60). Taxon age is positively correlated with duration (52), which is the reciprocal of extinction rate. Lower extinction rates at high latitudes imply that selection for generalism and vagility buffers extinction effectively (Fig. 3). Shorter-lived species rule out lower extinction rates as the cause for the high tropical regional diversity. If so, the cause for the latitudinal diversity gradient must be differential gradual speciation rates, in turn probably caused by differential ORD. This does not mean that tropical diversification must always be higher. Small ORD may, in the long run, increase extinction rates by allowing specialization and low vagility to evolve and by decreasing species' range sizes (Fig. 1). However, in the long-term absence of differential diversification, interbiome invasions would ultimately erode the diversity gradient.
Species Range Sizes.
ORD increases the geographical ranges of species (Fig. 1, Box 6). First, species with wide distributions are more likely to survive during climatic shifts (5). In the fossil record, species with large range sizes are generally less extinction-prone (49, 52). Second, generalist species find habitats over large geographical areas. Third, vagile and generalized species attain larger range sizes through effective colonization of regions with suitable habitats and by maintaining populations in sink areas. In many taxa, species with more effective dispersal range more widely (52, 54). Fourth, as species' ranges are changed geographically, populations may persist locally in regions that have become generally hostile either as enduring, long-lived individuals or in microclimatically suitable sites. Large ranges is another ORD-induced factor augmenting extinction resistance (Fig. 1, Boxes 6 and 8b).
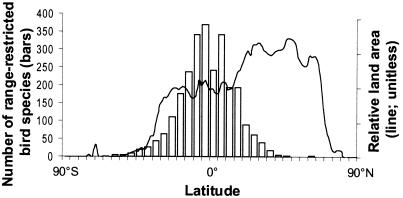
A latitudinal gradient with smaller ranges in the tropics is well established for several taxa (61) and has even been assigned the status of a rule, Rapoport's rule, although its generality has been questioned (62). On a global scale, distribution patterns are best known for birds. Only six of the 2,623 most range-restricted birds in the world (breeding range <50,000 km2) occur north of 45°N (63), despite the fact that one third of the ice-free land of the world is north of this latitude (Fig. 4). The global distribution of areas with range-restricted birds correlates well with patterns in other terrestrial taxa. For example, 70% of the “Centres of Plant Diversity” (indicating concentrations of plants with narrow distributions) identified in the world overlap with an “Endemic Bird Area” (63). Hence, the proportion of plant species with small ranges (<50,000 km2) is higher in tropical than temperate floras (64). In boreal and arctic areas, such narrowly endemic plant species are very few. The few plant taxa with small ranges found in Quaternary glaciated areas are mainly subspecies, products of abrupt speciation involving hybridization, and probably of very recent origin (65).
Figure 4.
Larger ORD toward the poles causes a gradient in species' range size. Bird species with small breeding ranges (<50,000 km2) are concentrated around the equator and lacking in vast high-latitude land areas in the Northern Hemisphere. Bars show the global number of range-restricted bird species (2,623 or 27% of all bird species) per 5° latitudinal band. Data from Stattersfield et al. (63). The line represents the latitudinal distribution of ice-free land, adapted from Rosenzweig (71).
Rapoport's rule should be more prominent during warmer periods when formerly glaciated and polar-desert areas are colonized by species with populations still persisting in lower-latitude areas (13). Larger range sizes at high latitudes further reduce extinction rates toward the poles (Fig. 3).
Abrupt Speciation.
Abrupt speciation mostly takes place as polyploidization, but also diploid hybrid speciation occurs. Several aspects of polyploid speciation make it more frequent in regions with large ORD (Fig. 1, Box 7). First, large ORD implies that few species are specialized to contemporary environments. Hybrid polyploids may rapidly provide reproductively isolated populations with new genetic combinations better adapted to new, suddenly appearing habitats (66–68). Consequently, polyploids are more frequently found in habitats being recently human made or most modified by recent climatic change (66, 69). Second, during periods of large and rapid climatic change, a new hybrid species can expand its distribution and avoid being competitively excluded by its parents. Third, ORD makes previously separated populations more likely to encounter and produce polyploids (66, 67). Fourth, apomixis is generally associated with polyploidy, and facilitates long-distance dispersal, because one individual is sufficient for establishment and reproduction. Consequently, apomictic representatives of clades more often range into previously glaciated areas (70). Good colonizing ability should lead to wide distributions, and there are many examples of temperate/arctic clades of plants where diploids have small and polyploids wide distributions (66). Fifth, the raw material of polyploid speciation is taxa with lower chromosome numbers produced by gradual speciation. As argued above, ORD may lead to lower extinction and gradual speciation rates, and consequently older species, which have had longer time to produce polyploids leading to higher proportions of polyploids.
The proportion of polyploids increases with latitude in all taxa studied. Polyploidy in seed plants (71) and freshwater zooplankton (72) as well as the frequency of high ploidy levels in mosses (73) increase with latitude. However, in northwestern North America, there is an inverted latitudinal gradient. The highest proportion of polyploid plant species is in formerly glaciated areas (larger ORD) to the south of the permanently ice-free northern Alaska (smaller ORD), where diploids as well as range-restricted species are more frequent (66, 67).
Spatial and Temporal Patterns.
Differential ORD can potentially explain many evolutionary patterns. Spatially, we may compare any pair of regions differing in the degree of ORD. There are two replicates of the latitudinal gradient with one in each hemisphere. Climatic oscillations have been smaller in the Southern Hemisphere (6, 7) (Fig. 2B). Consequently, several taxa, e.g., spider species and termite genera (74, 75), have smaller range sizes at equal latitudes in the Southern Hemisphere. These and other taxa (76) are also more diverse in the Southern Hemisphere, which can be explained by higher gradual speciation rates caused by smaller ORD. The number of range-restricted bird species is lower at mid latitudes in the Northern Hemisphere (Fig. 4). It has even been argued that Rapoport's rule is confined to mid and high latitudes of the Northern Hemisphere (62).
Lakes are generally ephemeral on a geological time scale. This selects for vagility, and most lake species are good dispersers speciating slowly (77). However, in freshwater systems that have persisted for long geological periods, such as Lakes Baikal and Tanganyika, populations have been long lived and nonvagile species have evolved. Such clades typically form radiating species flocks, whereas vagile taxa are nonspeciose even in these lakes (77). Despite the high-latitude position of Lake Baikal, many taxa persisted locally during the Quaternary climatic oscillations. The pelagic and deep benthic habitats harbor species flocks of both ancient, endemic lineages and recently immigrated taxa presently undergoing radiation (78). This high diversity and endemism contrast sharply with other large but recently glaciated or geologically young boreal lakes having few and widespread species.
Over millions of years, the amplitude of climatic oscillations changes. When entering periods with larger ORD, the immediate effect is falling gradual speciation and rising extinction rates, resulting in declining diversification. With time, selection results in less specialized, more vagile organisms and larger geographical range sizes. This slows down speciation further but mitigates extinction. When entering a subsequent period with lower amplitude, opposite temporal patterns should unfold. However, changes in the amplitude of Milankovitch climatic oscillations are often accompanied by changes in the longer-term mean climate (over millions of years). Such changes in mean climate are claimed to cause periods of increased speciation and extinction, so-called turnover pulses (79). We propose that changes in the long-term mean climate increases gradual speciation because more populations become isolated under new conditions long enough to complete speciation, compared with the short-term isolations caused by ORD. Moreover, the size and location of species' ranges are altered both by changes in the mean climate and by short-term oscillations, the cumulative effect of which causes increased species extinction. Turnover pulses coinciding with changes in the amplitude of ORD may temporarily reinforce or dampen the effect of the latter. For example, the larger climatic oscillations during the Quaternary compared with the preceding Pliocene epoch were accompanied by an average cooling (4) potentially producing a turnover pulse. The empirical data from these epochs are consistent with the above scenarios, and also imply that isolate persistence rather than isolate formation is the limiting factor in gradual speciation. Marine ostracodes radiated after the general climatic cooling. During the subsequent high-amplitude climatic oscillations, no new species arose, probably because of extinction of recurrently formed incipient species (39). The net diversification rate assessed from phylogenies of North American passerine birds was lower in the Quaternary (larger ORD) compared with the Pliocene (smaller ORD) (80). Furthermore, molecular studies have shown that a majority of vertebrate speciations suggested to be of late Quaternary origin were initiated before the Quaternary (81, 82).
In the Cape Floristic Region of Southern Africa, the Quaternary climate has been comparatively stable (Fig. 2B), with little ORD (83). This region has extremely high vascular plant diversity produced recently by high speciation rates, as well as many plants with narrow habitat specializations and small geographical ranges (84, 85). Bryophyte endemism is exceptionally high, with the ranges of more than 40% of the hepatic species confined to Southern Africa (86). In the low-productive mediterranean shrublands of the Cape called fynbos, small ORD has relaxed the selection against specialization and traits associated with poor dispersal, and most plant species disperse their seeds only a few meters (84). Among closely related taxa, the ones with low seed dispersability have diversified more, suggesting that low gene flow is a major factor in the radiations (84). Within the Cape flora, the strong diversification and the associated characteristics of species are not confined to the fynbos, but are equally pronounced in the adjacent Succulent Karoo having a different flora (85). The Succulent Karoo is drier but most probably experienced small Quaternary ORD as well. Polyploidy is considered to be infrequent in the Cape flora, although no comprehensive study has been made (87). Species-rich fynbos taxa such as Erica, Aspalathus, and Proteaceae have no recorded polyploid species (87). To conclude, the Cape flora exhibits five character states that we predict for the species pool of a region with small ORD: high specialization, low vagility, high gradual speciation rates, small range sizes, and low proportions of species formed by abrupt speciation (Fig. 1). Hence, two phenomena as different as latitudinal gradients and the unique Cape flora show the same character correlations and can be explained by differential ORD.
Conclusions
Differential magnitudes of ORD parsimoniously explain, with a single driving force, several biological phenomena that up to now have been given separate explanations. Historical explanations are often viewed as pertaining to unique events, not being general. ORD is a highly general, recurrent process providing innumerable spatial, temporal, and phylogenetic replicates. The phylogenetic replicates consist of sister clades that have experienced different magnitudes of ORD.
By taking the concepts and data of paleoecologists and paleontologists into account, ecologists and microevolutionary biologists could shed new light on old questions. In this way, more phenomena may be studied at their proper spatiotemporal scale.
The Current Extinction Crisis.
Most species have experienced many Milankovitch oscillations, showing that they may have the potential to survive future human-induced climatic changes. However, most species have survived by tracking their habitats through space, which is becoming increasingly difficult as humans have come to dominate and transform most of the ecosystems of the Earth (14). Furthermore, the insular habitats produced by humanmade fragmentation strongly select against vagility if the matrix is hostile.
Differential ORD and its evolutionary consequences call for new conservation strategies on the regional to global scale. Strategies may not be interchangeable among regions. Regions with a history of small ORD are often hotspots of species diversity, and harbor many species with high specialization, low vagility, and small geographical ranges. These traits render them vulnerable to climatic change and habitat destruction, making conservation a more arduous and costly task. In contrast, species having experienced large ORD should be more resilient to many human disturbances (14). Moreover, as humans make habitat tracking increasingly difficult, areas where global climatic change is buffered against and/or where steep environmental gradients reduce the need for migration, become more important for species' survival. To conclude, we suggest the use of characters related to small ORD [high climatic stability (88) or steep environmental gradients] in identifying both regional and global priority areas for conservation. This strategy could be of great importance because patterns in diversity and characters of species are well known only for a few taxa and some regions.
Acknowledgments
We thank G. Arnqvist, K. D. Bennett, A. G. Fischer, K. Hylander, E. G. Leigh, Jr., A. N. Nilsson, C. Nilsson, R. E. Ricklefs, and A. Saura for improving the manuscript with their comments, and A. J. Weaver for providing us with Fig. 2B. E. Carlborg and M. Svedmark helped with data and reference management. M.D. thanks the Swedish Council for Forestry and Agricultural Research.
Abbreviations
- ORD
orbitally forced species' range dynamics
- kyr
thousand years
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berger A. Rev Geophys. 1988;26:624–657. [Google Scholar]
- 2.Berger A. In: Climate and Geo-Sciences. Berger A, Schneider A, Duplessy J C, editors. Dordrecht: Kluwer; 1989. pp. 47–76. [Google Scholar]
- 3.Stanley S M. Paleobiology. 1985;11:13–26. [Google Scholar]
- 4.Webb T, Bartlein P J. Annu Rev Ecol Syst. 1992;23:141–173. [Google Scholar]
- 5.Bennett K D. Evolution and Ecology. Cambridge, U.K.: Cambridge Univ. Press; 1997. [Google Scholar]
- 6.Imbrie J, McIntyre A, Mix A. In: Climate and Geo-Sciences. Berger A, Schneider A, Duplessy J C, editors. Dordrecht: Kluwer; 1989. pp. 121–164. [Google Scholar]
- 7.Kutzbach J E, Guetter P J, Behling P J, Selin R. In: Global Climates Since the Last Glacial Maximum. Wright H E, Kutzbach J E, Webb T III, Ruddiman W F, Street-Perrott F A, Bartlein P J, editors. Minneapolis: Univ. Minnesota Press; 1993. pp. 24–93. [Google Scholar]
- 8.Imbrie J, Berger A, Boyle E A, Clemens S C, Duffy A, Howard W R, Kukla G, Kutzbach J, Martinson D G, McIntyre A, et al. Paleoceanography. 1993;8:699–735. [Google Scholar]
- 9.Weaver A J, Eby M, Augustus F F, Wiebe E C. Nature (London) 1998;394:847–853. [Google Scholar]
- 10.Bard E. Science. 1999;284:1133–1134. [Google Scholar]
- 11.Cronin T M, Raymo M E. Nature (London) 1997;385:624–627. [Google Scholar]
- 12.Huntley B, Birks H J B. An Atlas of Past and Present Pollen Maps for Europe: 0–13,000 Years Ago. Cambridge, U.K.: Cambridge Univ. Press; 1983. [Google Scholar]
- 13.Bennett K D, Tzedakis P C, Willis K J. J Biogeogr. 1991;18:103–115. [Google Scholar]
- 14.Coope G R. In: Extinction Rates. Lawton J H, May R M, editors. Oxford: Oxford Univ. Press; 1995. pp. 55–74. [Google Scholar]
- 15.Bush M B, Colinvaux P A. J Veg Sci. 1990;1:105–118. [Google Scholar]
- 16.Bush M B, Colinvaux P A, Wiemann M C, Piperno D R, Liu K B. Quat Res. 1990;34:330–345. [Google Scholar]
- 17.Colinvaux P A, De Oliveira P E, Moreno J E, Miller M C, Bush M B. Science. 1996;275:85–88. [Google Scholar]
- 18.Hewitt G. Biol J Linn Soc. 1996;58:247–276. [Google Scholar]
- 19.Cwynar L C, MacDonald G M. Am Nat. 1987;129:463–469. [Google Scholar]
- 20.Futuyma D J. In: Speciation and Its Consequences. Otte D, Endler J A, editors. Sunderland, MA: Sinauer; 1989. pp. 557–578. [Google Scholar]
- 21.Otte D. In: Speciation and Its Consequences. Otte D, Endler J A, editors. Sunderland, MA: Sinauer; 1989. pp. 482–526. [Google Scholar]
- 22.Nilsson A N, Pettersson R B, Lemdahl G. J Biogeogr. 1993;20:227–234. [Google Scholar]
- 23.Scriber M J. Psyche. 1973;80:355–373. [Google Scholar]
- 24.Taylor J D, Taylor C N. J Biogeogr. 1977;4:73–81. [Google Scholar]
- 25.Karr J R. In: Biogeography and Ecology of Forest Bird Communities. Keast A, editor. The Hague: SPB Academic Publishing; 1990. pp. 215–228. [Google Scholar]
- 26.Pagel M D, May R M, Collie A R. Am Nat. 1991;137:791–815. [Google Scholar]
- 27.Whittaker R H. Ecology. 1954;35:275–288. [Google Scholar]
- 28.Lord J, Egan J, Clifford T, Jurado E, Leishman M, Williams D, Westoby M. J Biogeogr. 1997;24:205–211. [Google Scholar]
- 29.Lawrey J D. Bryologist. 1980;83:344–350. [Google Scholar]
- 30.Sipman H J M, Harris R C. In: Tropical Rain Forest Ecosystems. Biogeographical and Ecological Studies. Lieth M, Werger M J A, editors. Amsterdam: Elsevier; 1989. pp. 303–309. [Google Scholar]
- 31.Ryvarden L. In: Aspects of Tropical Mycology. Isaac S, editor. Vol. 19. Cambridge, U.K.: Cambridge Univ. Press; 1993. pp. 149–170. [Google Scholar]
- 32.Diamond J M. In: Extinctions. Nitecki M H, editor. Chicago: Univ. Chicago Press; 1984. pp. 191–246. [Google Scholar]
- 33.Poulin E, Féral J-P. Evolution (Lawrence, Kans) 1996;50:820–830. doi: 10.1111/j.1558-5646.1996.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 34.Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard Univ. Press; 1963. [Google Scholar]
- 35.Allmon W D. In: Oxford Surveys in Evolutionary Biology. Futuyma D, Antonovics J, editors. Vol. 8. Oxford: Oxford Univ. Press; 1992. pp. 219–257. [Google Scholar]
- 36.Coyne J A, Orr H A. Evolution (Lawrence, Kans) 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- 37.Avise J C, Walker D, Johns G C. Proc R Soc London Ser B. 1998;265:1707–1712. doi: 10.1098/rspb.1998.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schluter D. Philos Trans R Soc London B. 1996;351:807–814. [Google Scholar]
- 39.Cronin T M. Science. 1985;227:60–63. doi: 10.1126/science.227.4682.60. [DOI] [PubMed] [Google Scholar]
- 40.Williamson P G. Nature (London) 1981;293:437–443. [Google Scholar]
- 41.Williams G C. Natural Selection. New York: Oxford Univ. Press; 1992. [Google Scholar]
- 42.Potts E C. Bull Mar Sci. 1983;33:619–632. [Google Scholar]
- 43.Fjeldsa J, Lovett J C. Biodiv Conserv. 1997;6:325–346. [Google Scholar]
- 44.Konnert M, Bergmann F. Plant Syst Evol. 1995;196:19–30. [Google Scholar]
- 45.Glazier D S. J Theor Biol. 1987;126:323–334. doi: 10.1016/j.jtbi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 46.McGlone M S. Global Ecol Biogeogr. 1996;5:309–314. [Google Scholar]
- 47.Coope G R. Philos Trans R Soc London B. 1986;314:619–635. [Google Scholar]
- 48.Agakhanjanz O, Breckle S-W. In: Arctic and Alpine Biodiversity. Chapin F S, Körner C, editors. Berlin: Springer; 1995. pp. 63–80. [Google Scholar]
- 49.Jablonski D. Bull Mar Sci. 1986;39:565–587. [Google Scholar]
- 50.Jablonski D. In: Extinction Rates. Lawton J H, May R M, editors. Oxford: Oxford Univ. Press; 1995. pp. 25–42. [Google Scholar]
- 51.Chown S L. In: The Biology of Rarity. Kunin W E, Gaston K J, editors. London: Chapman & Hall; 1997. pp. 91–107. [Google Scholar]
- 52.Stanley S M. Macroevolution. San Francisco: Freeman; 1979. [Google Scholar]
- 53.Vrba E S. Evol Ecol. 1989;1:283–300. [Google Scholar]
- 54.Gradstein S R, Pócs T. In: Tropical Forest Ecosystems. Biogeographical and Ecological Studies. Lieth H, Werger M J A, editors. Amsterdam: Elsevier; 1989. pp. 311–325. [Google Scholar]
- 55.Terborgh J. Diversity and the Tropical Rain Forest. New York: Scientific American Library; 1992. [Google Scholar]
- 56.Lynch J D. In: Speciation and Its Consequences. Otte D, Endler J A, editors. Sunderland, MA: Sinauer; 1989. pp. 527–553. [Google Scholar]
- 57.Stehli F G, Douglas R G, Newell N D. Science. 1969;164:947–949. doi: 10.1126/science.164.3882.947. [DOI] [PubMed] [Google Scholar]
- 58.Fischer A G. Evolution (Lawrence, Kans) 1960;14:64–81. [Google Scholar]
- 59.Cardillo M. Proc R Soc London Ser B. 1999;266:1221–1225. [Google Scholar]
- 60.Flessa K W, Jablonski D. In: Evolutionary Paleobiology. Jablonski D, Erwin D H, Lipps J H, editors. Chicago: Univ. Chicago Press; 1996. pp. 376–397. [Google Scholar]
- 61.Lawton J H. Philos Trans R Soc London B. 1994;344:61–68. [Google Scholar]
- 62.Gaston K J. Oikos. 1999;84:309–312. [Google Scholar]
- 63.Stattersfield A J, Crosby M J, Long A J, Wege D C. Endemic Bird Areas of the World. Cambridge, U.K.: BirdLife International; 1997. [Google Scholar]
- 64.Gentry A H. In: Conservation Biology. Soulé M E, editor. Sunderland, MA: Sinauer; 1986. pp. 153–181. [Google Scholar]
- 65.Jonsell B. Blyttia. 1990;48:79–81. [Google Scholar]
- 66.Stebbins G L. Chromosomal Evolution in Higher Plants. London: Edward Arnold; 1971. [Google Scholar]
- 67.Stebbins G L. Bot Helv. 1984;94:1–14. [Google Scholar]
- 68.Brochmann C, Elven R. Evol Trend Plant. 1992;6:111–124. [Google Scholar]
- 69.Hodgson J G. Funct Ecol. 1987;1:243–250. [Google Scholar]
- 70.Bierzychudek P. Experientia. 1985;41:1255–1264. [Google Scholar]
- 71.Rosenzweig M L. Species Diversity in Space and Time. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 72.Ward R D, Bickerton M A, Finston T, Hebert P D N. Heredity. 1994;73:532–543. [Google Scholar]
- 73.Kuta E, Przywara L. Acta Biol Cracov Bot. 1997;39:17–26. [Google Scholar]
- 74.Platnick N I. J Nat Hist. 1991;25:1083–1088. [Google Scholar]
- 75.Eggleton P. J Nat Hist. 1994;28:1209–1212. [Google Scholar]
- 76.Clarke A, Crame J A. In: Marine Biodiversity: Patterns and Processes. Ormond R F G, Gage J D, Angel M V, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 122–147. [Google Scholar]
- 77.Cohen A S, Johnston M R. Palaios. 1987;2:426–435. [Google Scholar]
- 78.Sherbakov D Y. Trends Ecol Evol. 1999;14:92–95. doi: 10.1016/s0169-5347(98)01543-2. [DOI] [PubMed] [Google Scholar]
- 79.Vrba E S. Am J Sci. 1993;293A:418–452. [Google Scholar]
- 80.Zink R M, Slowinski J B. Proc Natl Acad Sci USA. 1995;92:5832–5835. doi: 10.1073/pnas.92.13.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klicka J, Zink R M. Science. 1997;277:1666–1669. [Google Scholar]
- 82.Bates J M, Hackett S J, Cracraft J. J Biogeogr. 1998;25:783–793. [Google Scholar]
- 83.Meadows M E, Sugden J M. Palaeogeogr Palaeoclimatol Palaeoecol. 1993;101:271–281. [Google Scholar]
- 84.Goldblatt P. Biodiv Conserv. 1997;6:359–377. [Google Scholar]
- 85.Cowling R M, Rundel P W, Desmet P G, Esler K J. Div Distrib. 1998;4:27–36. [Google Scholar]
- 86.Schofield W B. In: Bryophytes and Lichens in a Changing Environment. Bates J W, Farmer A M, editors. Oxford: Clarendon; 1992. pp. 103–130. [Google Scholar]
- 87.Goldblatt P. Ann Missouri Bot Gard. 1978;65:369–436. [Google Scholar]
- 88.Fjeldsa J, Ehrlich D, Lambin E, Prins E. Biodiv Conserv. 1997;6:401–422. [Google Scholar]